Abstract
The genome of bovine adenovirus type 3 (BAV3) is flanked by 195 base pair inverted terminal repeats (ITR). We isolated a BAV3 mutant (BAV3c29) having an internal deletion within the left ITR. The deletion eliminated 72 bp between nucleotides nt 89 and 162, including most of the GC-rich sequences located close to the end of the ITR sequences. This deletion did not seem to have any affect on the virus plaque size or morphology and the kinetics of viral replication compared to wild-type (wt) BAV3. The nt sequence of the right ITR of BAV3c29 remained identical to the right or left ITR of wt BAV3. These results indicate that the cis-acting sequences present within the 72 bp between nt 89 and 162 of the left ITR are not essential for BAV3 DNA replication in cultured cells.
Keywords: Bovine adenovirus type 3 inverted terminal repear deletion, adenovirus inverted terminal repeat, adenovirus recombinant
The bovine adenovirus type 3 (BAV3) genome is a linear double-stranded DNA molecule of 34,446 base pairs (bp) [1], flanked by 195 bp inverted terminal repeats (ITR) [2]. The presence of these terminal redundancies has been described for all known adenoviruses [2-7]. The left and the right ITRs are of the abc-c'b'a' conformation, with lengths ranging from 52 bp in the avian egg drop syndrome -76 adenovirus [2] to 238 bp in the porcine adenovirus type 4 [4].
The terminal protein (TP) is attached to the 5' -OH terminus of the genome by a phosphodiester bond [8]. The precursor of the terminal protein covalently binds to the first deoxycytidylyl nucleotide residue (dCMP) of the newly synthesized DNA chain [8]. The protein-bound dCMP then functions as a primer for DNA synthesis.
The ITR nucleotide (nt) sequences that are important in human adenovirus replication include the origin of replication (ori) represented by the first 18 or 21 nt and an auxiliary region that extends from nt 36 up to 67 [9]. These two regions contain binding sites for the origin recognition protein (ORP-A), nuclear factor (NF) I (NFI) and NFIII. Between nt 51 and 103, the ITRs of human and simian adenoviruses also contain consensus motifs known as ‘GC box’ and 5'-TGACGT-3' for the binding of SP1 and ATF, respectively.
The boundaries of the minimal cis-acting sequences in the ITR and the factors involved in viral DNA replication have been extensively studied for human adenoviruses, whereas only sequence comparisons and phylogenetic analyses have been reported for the ITRs of non-human adenoviruses. This short communication describes a 72 bp internal deletion in the left ITR of BAV3 that does not seem to affect virus replication in cultured cells.
Homologous recombination in bacteria is a powerful tool employed for the construction of human and non-human adenovirus recombinants [1, 10-12]. We have used this method for the cloning of the full-length BAV3 genome [13], and recently we found out that PCR amplification of the left end of the genome generated a BAV3c29 mutant (fig. 1A). Initial comparison of DNA fragments generated by the EcoRI digestion of genomic DNA of wild-type (wt) BAV3 and BAV3c29 showed that the expected left end fragment for BAV3c29 was slightly smaller than that produced by the wt BAV3 genome (fig. 1B). The discrepancy in the size of the left end fragment was further confirmed by AscI digestion. The finding of a smaller than expected fragment corresponding to the left end of the BAV3c29 genome prompted us to locate the deletion by DNA sequencing. Nucleotide sequence analyses of the left ITR regions of wt BAV3, BAV3c29 and two plasmids containing the ITR of BAV3c29, pMvOBE1E4PacI [13] and pMvOBAV3c29, revealed a 72 bp deletion including nt 91 through 161 of the left ITR of pMvOBE1E4PacI, pMvOBAV3c29 and BAV3c29. The left ITR of wt BAV3 was without any deletion. Interestingly, the right ITRs in wt BAV3, pMvOBE1E4PacI, pMvOBAV3c29 and BAV3c29 were intact without any compensating deletion. In addition to the 72 bp deletion, the left ITR of BAV3c29 also had nt substitutions at positions 170 (G to T), 172 (G to T) and 173 (C to T). A detailed description and the specific regions of the ITR affected by these mutations are presented figure 2. The left end of wt BAV3 between 1 and 1156 nt was amplified using the same primers described in the legend to figure 1, with a number of changes in the PCR reaction conditions. On the basis of restriction enzyme digestion and agarose gel electrophoresis, all PCR products were slightly shorter than the expected fragment containing the left ITR (data not shown). Our results of restriction enzyme digestion and sequence analyses of wt BAV3, BAV3c29, pMvOBE1E4Pac1 and pMvOBAV3c29 indicated that the internal deletion in the left ITR was due to PCR amplification.
Fig. 1.

Generation of BAV3c29 and restriction enzyme analysis of genomic DNA extracted from wt BAV3 and BAV3c29. A) Diagram representing the generation of the internal deletion in the left ITR during PCR amplification of the EcoRI fragment of BAV3. PCR was performed using an oligonucleotide encoding the PacI recognition site and the first 21 bp of the BAV3 ITR (PacI-1-21) as a forward primer and the nucleotidet sequence from 1138 to 1159 as a reverse primer. The purified PCR product was subsequently cloned in pUC18 to form pMvOBE1E4PacI (van Olphen & Mittal, 1999), and it was subsequently then used with the BAV3 genome to generate plasmid pMvOBAV3c29 by recombination in Escherichia. coli BJ5183. Genome-length DNA obtained from pMvOBAV3c29 was linearized with PacI and transfected into MDBK cells using a lLipofectin-mediated transfection protocol (Life Technologies). Two weeks post-after transfection, an isolated viral plaque was collected and propagated to produce the BAV3c29 stock. The left-end AscI fragment of wt BAV3, pMvOBAV3c29, and BAV3c29 depicting the ntucleotide position of the ITR boundaries, and the locations of EcoRI and AscI recognition sites are shown. ITRs are shown as open-boxes and the position and length of the internal ITR deletion are also shown. B) DNA samples obtained from purified preparations of wt BAV3 and BAV3c29 were digested with restriction enzymes, AscI and EcoRI. The resultant DNA fragments were loaded onto a 1.5% agarose gel andel, separated by electrophoresis and the ethidium bromide-stained bands were visualized with an UV transilluminator. The sizes of the terminal fragments containing the left ITR generated with the digestion of AscI and EcoRI are shown on the left and right side of the panel, respectively. MWM =, 1 kb DNA molecular weight marker.
Fig. 2.
Nucleotide sequence comparison of the left ITR of wt BAV3, BAV3c29 and HAV5. On the basis of sequence similarities with HAV5, the potential binding sites for a number of transcription factors were identified in the left BAV3 ITR. The potential binding motifs for ORP-A, NFI, NFIII, SP1 and ATF are depicted as gray underlined, underlined, double underlined, gray background and thick underlined, respectively. The deleted nt in the left ITR of BAV3c29 are depicted by the symbol ‘∼’. The conserved nt in the binding motif for NFI are shown in bold. The nt substitutions in BAV3c29 are shown in bold face with lines above and below.
The first 13 bp of the BAV3 ITR are identical to their HAV5 counterpart and may represent the binding site for ORP-A (Fig. 2). A potential binding motif for NFI, TGGN12GCCAAT is located between 27 and 49 nt. In the BAV3 ITR, the sequences recognized by NFIII do not seem to be present with the same nt composition as in HAV5 ITR; however between 68 and 80 nt, there may be a potential NFIII recognition site. The ITR of BAV3 contains two potential ATF binding sites from 70 to 105 nt and five consensus SP1 binding sites from 142 to 195 nt. The left ITR of BAV3c29 (123 bp long) is still 20 bp longer than that of the HAV5 ITR (103 bp long). The 72-bp deletion removed at least three consensus SP1-binding sites and one potential ATF binding site; thereby, the remaining two SP1 sites came in to closer proximity to the auxiliary region of the left BAV3 ITR.
We also examined the ability of BAV3c29 to replicate in MDBK cells and compared its growth characteristics with those of wt BAV3. The time needed for plaque formation after infection and the size and morphology of plaques were similar to those produced by wt BAV3 (fig. 3). To determine whether the deletion in the left ITR of BAV3c29 affects virus replication, the kinetics of BAV3c29 replication was compared with that of wt BAV3 in MDBK cells infected at an MOI of 2 PFU per cell (fig. 4). The replication kinetics and virus titers of BAV3c29 seemed to be similar to that of wt BAV3. To quantitate the total amount of DNA incorporated into newly synthesized virions of mutant compared to wt BAV3, a fixed number of MDBK cells were infected with BAV3c29 or wt BAV3 at a MOI of 5 PFU per cell, and virus-infected cells were harvested 48 h after infection. The viral samples were purified by cesium chloride density gradient centrifugation, and the viral DNA from purified virions was extracted using an SDSpronase method [14]. The DNA concentration was monitored by measuring optical density at 260 nm using a spectrophotometer and also by restriction enzyme analysis. Similar amounts of DNA were recovered from purified preparations of BAV3c29 and BAV3 (data not shown) suggesting that BAV3c29 DNA replication was not affected.
Fig. 3.
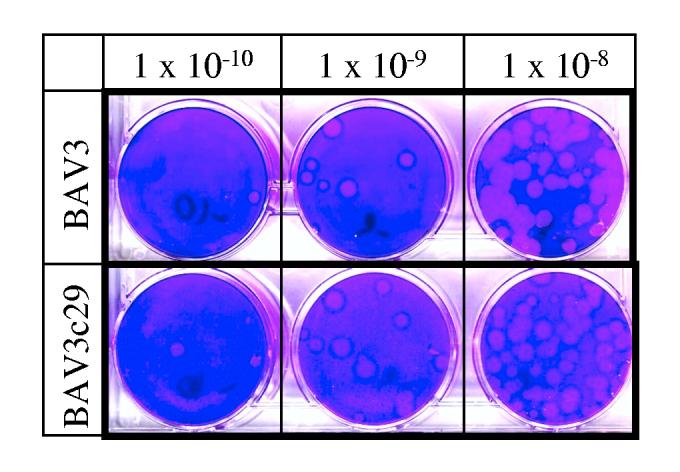
Development of plaques by BAV3 and BAV3c29 on MDBK cells. Confluent monolayers of MDBK cells in 60 mm dishes were infected with various log dilutions of either BAV3 or BAVc29 and the virus was allowed to adsorb for 1 h at 37°. Subsequently monolayers were covered with an agarose overlay (1 x MEM, 5% fetalCloneIII, 0.5% agarose, 0.05% yeast extract, 50 μg/ml gentamicin and 25mM MgCl2). Plaques were stained with crystal violet 12 days after infection. Dishes infected with virus dilutions of 10-8, 10-9 and 10-10 are shown.
Fig. 4.
Kinetics of replication of wt BAV3 and mutant BAV3c29. MDBK cell monolayers were grown in 60mm culture dishes and infected with wt BAV3 or BAV3c29 at an MOI of 2 PFU per cell. Infected cells along with the medium were collected 6, 12, 24, 48, 72 and 96 h after infection and the virus titration was done by plaque assay on MDBK cells.
The elimination of the 72-bp internal deletion in the BAV3 left ITR did not seem to affect the replication of the viral DNA, probably due to the following reasons. Since both the left and right ori are intact, replication of the first strand from each end may not be affected. The first 89 nt that are complementary between opposite ITRs may be sufficient for the formation of the panhandle structure, therefore, synthesis of the second strand by type II replication [15] is not impaired. The deletion did not remove the auxiliary region of the ori, and at least one potential ATF-binding site and two Sp1-binding sites were intact.
It has been demonstrated that complete deletion of the ITR of adenoviruses resulted in non-infectious DNA [16, 17]. Flanking of ITR sequences rendered the ori inactive, and consequently, infectious progeny virions were not produced, indicating that the presence of a free terminal ori is essential for initiation of DNA replication [18]. The formation of the panhandle in HAV5 requires a minimum of the first 31 complementary nt of the ITR [19]. In contrast, deletions within the left ITR of HAV2 starting beyond nt 45 were stable and did not affect virus replication [16]. A virus carrying a deletion including the NFIII, Sp1 and ATF sites within the ITR replicated to similar titers as those obtained with wt HAV5 [20], thereby suggesting that there is functional redundancy between different transcription factors that interact with the left ITR of the HAV5 genome.
Further mutational and functional analyses are required to precisely define the minimal ITR sequences that are necessary for BAV3 replication and to identify the exact positions of binding motifs for various transcription factors. Our results support the exploration of this site as a novel location for the insertion of foreign genes.
Acknowledgements
This work was supported by Public Health Service grant GM5516 from NIH/NIGMS to S.K.M.
REFERENCES
- 1.Reddy PS, Idamakanti N, Chen Y, Whale T, Babiuk LA, Mehtali M, Tikoo SK. Replication defective bovine adenovirus type 3 as an expression vector. J Virol. 1999;73:9137–9144. doi: 10.1128/jvi.73.11.9137-9144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shinagawa M, Iida Y, Matsuda A, Tsukiyama T, Sato G. Phylogenetic relationships between adenoviruses as inferred from nucleotide sequences of inverted terminal repeats. Gene. 1987;55:85–93. doi: 10.1016/0378-1119(87)90251-4. [DOI] [PubMed] [Google Scholar]
- 3.Alestrom P, Stenlund A, Li P, Pettersson U. A common sequence in the inverted terminal repetitions of human and avian adenoviruses. Gene. 1982;18:193–197. doi: 10.1016/0378-1119(82)90117-2. [DOI] [PubMed] [Google Scholar]
- 4.Reddy PS, Tuboly T, Dennis JR, Derbyshire JB, Nagy E. Comparison of the inverted terminal repetition sequences from five porcine adenovirus serotypes. Virology. 1995;212:237–239. doi: 10.1006/viro.1995.1475. [DOI] [PubMed] [Google Scholar]
- 5.Zheng B, Mittal SK, Graham FL, Prevec L. The E1 sequence of bovine adenovirus type 3 and complementation of human adenovirus type 5 E1A function in bovine cells. Virus Res. 1994;31:163–186. doi: 10.1016/0168-1702(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 6.Tokunaga O, Shinagawa M, Padmanabhan R. Physical mapping of the genome and sequence analysis at the inverted terminal repetition of adenovirus type 4 DNA. Gene. 1982;18:329–334. doi: 10.1016/0378-1119(82)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Brinckmann U, Darai G, Flugel RM. The nucleotide sequence of the inverted terminal repetition of the tree shrew adenovirus DNA. Gene. 1983;24:131–135. doi: 10.1016/0378-1119(83)90138-5. [DOI] [PubMed] [Google Scholar]
- 8.Desiderio SV, Kelly TJ., Jr Structure of the linkage between adenovirus DNA and the 55,000 molecular weight terminal protein. J Mol Biol. 1981;145:319–337. doi: 10.1016/0022-2836(81)90208-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Pearson GD. Adenovirus sequences required for replication in vivo. Nucleic Acids Res. 1985;13:5173–5187. doi: 10.1093/nar/13.14.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70:4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakhartchouk AN, Reddy PS, Baxi M, Baca-Estrada ME, Mehtali M, Babiuk LA, Tikoo SK. Construction and characterization of E3-deleted bovine adenovirus type 3 expressing full-length and truncated form of bovine herpesvirus type 1 glycoprotein gD. Virology. 1998;250:220–229. doi: 10.1006/viro.1998.9351. [DOI] [PubMed] [Google Scholar]
- 12.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Olphen AL, Mittal SK. Generation of infectious genome of bovine adenovirus type 3 by homologous recombination in bacteria. J Virol Methods. 1999;77:125–129. doi: 10.1016/s0166-0934(98)00145-1. [DOI] [PubMed] [Google Scholar]
- 14.Mittal SK, Prevec L, Babiuk LA, Graham FL. Sequence analysis of bovine adenovirus type 3 early region 3 and fibre protein genes. doi: 10.1099/0022-1317-73-12-3295. [DOI] [PubMed] [Google Scholar]; J Gen Virol. J Gen Virol. 1993;1992;7473(Pt 12):2825, 3295–3300. [Google Scholar]
- 15.Daniell E. Genome structure of incomplete particles of adenovirus. J Virol. 1976;19:685–708. doi: 10.1128/jvi.19.2.685-708.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay RT, McDougall IM. Viable viruses with deletions in the left inverted terminal repeat define the adenovirus origin of DNA replication. J Gen Virol. 1986;67:321–332. doi: 10.1099/0022-1317-67-2-321. [DOI] [PubMed] [Google Scholar]
- 17.Stow ND. The infectivity of adenovirus genomes lacking DNA sequences from their left hand termini. Nucleic Acids Res. 1982;10:5105–5119. doi: 10.1093/nar/10.17.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanahan D, Gluzman Y. Rescue of functional replication origins from embedded configurations in a plasmid carrying the adenovirus genome. Mol Cell Biol. 1984;4:302–309. doi: 10.1128/mcb.4.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Xu FY, Ahern KG, Pearson GD. Inverted repeats direct repair of adenovirus minichromosome ends. Virology. 1991;183:44–51. doi: 10.1016/0042-6822(91)90116-s. [DOI] [PubMed] [Google Scholar]
- 20.Hatfield L, Hearing P. The NFIII/OCT-1 binding site stimulates adenovirus DNA replication in vivo and is functionally redundant with adjacent sequences. J Virol. 1993;67:3931–3939. doi: 10.1128/jvi.67.7.3931-3939.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]




