Abstract
We studied the role of protein kinase C isoform PKCδ in ceramide (Cer) formation, as well as in the mitochondrial apoptosis pathway induced by anticancer drugs in prostate cancer (PC) cells. Etoposide and paclitaxel induced Cer formation and apoptosis in PKCδ-positive LNCaP and DU145 cells but not in PKCδ-negative LN-TPA or PC-3 cells. In contrast, these drugs induced mitotic cell cycle arrest in all PC cell lines. Treatment with Rottlerin, a specific PKCδ inhibitor, significantly inhibited drug-induced Cer formation and apoptosis in LNCaP cells, as did overexpression of dominant negative–type PKCδ. Overexpression of wild-type PKCδ had an opposite effect in PC-3 cells. Notably, etoposide induced biphasic Cer formation in LNCaP cells. The early and transient Cer increase resulted from de novo Cer synthesis, while the late and sustained Cer accumulation was derived from sphingomyelin hydrolysis by neutral sphingomyelinase (nSMase). Cer, in turn, induced mitochondrial translocation of PKCδ and stimulated the activity of this kinase, promoting cytochrome c release and caspase-9 activation. Furthermore, the specific caspase-9 inhibitor LEHD-fmk significantly inhibited etoposide-induced nSMase activation, Cer accumulation, and PKCδ mitochondrial translocation. These results indicate that PKCδ plays a crucial role in activating anticancer drug–induced apoptosis signaling by amplifying the Cer-mediated mitochondrial amplification loop.
Introduction
In various apoptotic phenomena, an elevation of the intracellular ceramide (Cer) levels occurs in response to a variety of apoptotic stimuli, including anticancer regimens (1). Numerous studies have suggested that lipid ceramide is relevant for apoptosis signaling in response to etoposide (2, 3) and paclitaxel (4). Moreover, a failure to generate ceramide has been associated with apoptosis resistance (5). However, the mechanisms that fail to generate Cer in apoptosis-resistant cells and convey activation signals to the enzyme responsible for Cer formation remain ill-defined.
A novel protein kinase C isoform, PKCδ, is implicated in apoptotic cell death, and its decreased protein expression is associated with tumor cell promotion (6). We reported recently that PKCδ protein expression decreased in androgen-independent prostate cancer (PC) cells in which PKCδ protein degradation is promoted by neuropeptide-meditated Src kinase activation (7). Notably, PKCδ has been implicated in the mediation of apoptosis in response to various anticancer agents such as etoposide (8) and paclitaxel (9). Recent studies show that a translocation of PKCδ onto mitochon-dria induces cytochrome c (cyt c) release and caspase activation (9–11). Cer has also been implicated as a primary mediator in signaling to the mitochondrial apoptosis pathway (12, 13). Importantly, PKCδ and Cer interact with each other. Several investigators have reported that increased Cer can induce PKCδ translocation in apoptotic cell death (14, 15), thus suggesting the primary role of Cer in the activation of PKCδ-mediated apoptotic pathway. In contrast, another study showed that a PKC activator 12-O-tetradecanoylphorbol-13-acetate (TPA), which induces apoptosis through PKCδ activation in PC cells (7, 16), promotes Cer formation in LNCaP PC cells (17), thus leading to the possibility that activated PKCδ may in turn promote Cer formation in apoptosis. Furthermore, a recent report in which Cer formation was secondary to anticancer drug–induced mitochondrial events (2) argues against Cer playing a primary role in signaling to the mitochondrial apoptosis pathway. As a result, the signaling involving PKCδ and Cer in mitochondrial apoptosis is complicated, and the precise mechanism remains unclear.
In the present study, we report that PKCδ is required for the amplification of Cer formation and apoptosis induced by anticancer drugs in PC cells. In addition, we herein present that etoposide induces a biphasic increase in Cer formation and that Cer formation in each phase is regulated by distinct mechanisms. We demonstrated that PKCδ mitochondrial translocation induced by an early Cer increase resulted in mitochondrial change followed by caspase-9 activation required for sustained Cer accumulation. These findings suggest that Cer activated by PKCδ might provide an amplifying signal to mitochondria in a feedback loop of caspase activation.
Methods
Cell culture and reagents.
PC cell lines were maintained in either RPMI1640 medium (LNCaP) or MEM (PC-3 and DU145) supplemented with 2 mM glutamine, 1% nonessential amino acids, 100 U/ml streptomycin and penicillin, and 10% FCS. LNCaP derivative LN-TPA was maintained in the above media containing 10 nM TPA for more than 3 months as described previously (7, 18). Etoposide and paclitaxel were provided from Bristol-Myers Squibb KK (Tokyo, Japan) Cer analogues and various inhibitors were purchased from Calbiochem-Novabiochem Corp. (La Jolla, California, USA).
Cell lysate preparation and isolation of mitochondrial and cytosol fractions.
Total cell lysates were prepared with RIPA buffer as described previously (19). For the isolation of mitochondrial and cytosol fractions, cells were collected in PBS and then pelleted by centrifugation. The cell pellets were resuspended in a buffer containing 25 mM Tris (pH 7.4), 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and 1 mM DTT. Protease inhibitors (10 μg of leupeptin/ml, 10 μg of aprotinin/ml, and 1 mM PMSF) and phosphatase inhibitors (10 μM Na3VO4) were added prior to lysis. Cells were homogenized ten times with a Dounce homogenizer (Wheaton Scientific Products, Millville, New Jersey, USA). Unlysed cells and nuclei were removed by centrifugation at 750 g for 10 minutes. The supernatant was centrifuged at 10,000 g for 30 minutes, and the resulting pellet was washed once with the same buffer and represents the mitochondrial-enriched fraction. The supernatant was further spun at 100,000 g for 1 hour, and the supernatant from this final centrifugation represents the cytosol fraction.
Immunoblotting.
Immunoblotting procedures were performed as described previously (19) using anti-PKCδ, anti–Bcl-2 (1:1,000; Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), anti–Bcl-xL, and anti–caspase-3 or anti–caspase-9 (1:1,000; New England Biolabs Inc., Beverly, Massachusetts, USA). For the detection of cyt c in cytosolic fractions, 20 μg of cytosolic fractions were separated on 15% SDS-PAGE. The gels were then transferred, and membranes were immunoblotted with anti–cyt c (1:500; Santa Cruz Biotechnology Inc.) and detected. To monitor the loading equality of each lane, membranes were also immunoblotted with anti–actin (1:3,000; Chemicon International, Temecula, California, USA) for total cell lysates and cytosolic fraction or with anti–HSP-60 (1:500; Santa Cruz Biotechnology Inc.) for mitochondrial fraction. Relative intensities of each band were measured by NIH image.
Overexpression of PKCδ and dominant negative type of PKCδ in PC cells.
Replication-deficient adenovirus vectors for PKCδ (Ax-PKCδ), dominant negative type (DN type) (K376A) of PKCδ (Ax-DNPKCδ), and a control vector for LacZ (Ax-LacZ) were constructed and transfected as described previously (20–22). Briefly, subconfluent PC cells were infected with adenovirus vectors at different moi’s, and the cells were incubated in the infection media (MEM with 2% L-glutamine and 100 μg/ml streptomycin) for 3 hours. After removing the virus, cells were further incubated for specific time periods.
PKCδ activity assay.
The equal amounts of protein (500 μg per sample) from treated PC cells were immunoprecipitated with anti-PKCδ Ab (2 μg per sample). The washed immunoprecipitates were incubated at room temperature with 40 μl of reaction buffer that contained 10 μM PKCδ substrate derived from PKCδ pseudosubstrate region (23), 20 mM Tris-HCl, pH 7.5, 1 mM CaCl2, 10 μM magnesium acetate, 1 μM TPA, 50 μg/ml phosphatidylserine (Sigma-Aldrich, St. Louis, Missouri, USA), 30 μM ATP, and 30 μCi of [γ-32P]ATP for 20 minutes. The reaction tube was centrifuged, and 20 μl of the supernatant was spotted on phosphocellulase disk sheets (Invitrogen Corp., Carlsbad, California, USA). The sheets were washed twice with 1% phosphoric acid and twice with distilled water, and samples were analyzed by liquid scintillation. The specific PKCδ activity was calculated by subtracting the nonspecific catalytic activity from the total catalytic activity and was expressed as fold increase compared with the control.
Cell cycle analysis and apoptosis assay.
Cell cycle analysis and apoptosis assay were performed by flow cytometry and/or fragmented DNA ELISA as described previously (7, 21). In cell cycle analysis, fragmented apoptotic nuclei are recognized by their subdiploid (sub-G1) DNA content. All experiments were performed at least three times in duplicate.
Quantitation of Cer and sphingomyelin.
Intracellular Cer and sphingomyelin (SM) levels were determined following metabolic labeling with [14C]serine as described (2, 24), with minor modifications. PC cells (106/ml) were incubated for 24 hours with [14C]serine (0.2 μCi/ml) in MEM containing 0.3% FCS, washed with PBS, and then treated for specific time periods. After cells were detached with trypsin, total lipids were extracted with 7 ml of chloroform/methanol (1:2 vol/vol), and the phases were separated using 20 mM acetic acid. The extracts were spotted on Silica Gel 60 thin-layer chromatography (TLC) plates (Whatman Japan KK, Tokyo, Japan) and chromatographed with chloroform/methanol/0.22% aqueous CaCl2 (60:35:5 by volume) or chloroform/methanol/H2O/25%NH4OH (50:45:2:1 by volume). Radioactive spots were evaluated with the Fujix BAS 2000 PhosphorImager system (Fuji Photo Film Co., Tokyo, Japan). The intensity of each Cer band was measured relative to total radioactivity in phosphatidylethanolamine, which remained unaltered upon stimulation and was expressed as a relative value to each control set to 1. All experiments were performed at least three times, and the data were substantiated with a diacylglycerol kinase assay.
Sphingomyelinase assay.
Cell protein extract (100 μg) was incubated with 10 nmol of [14C]SM (the specific radioactivity was adjusted to 10,000 cpm/nmol by the addition of unlabeled SM)/0.4 M Tris/HCl, pH 7.5/0.2% Triton X-100 in a final volume of 100 μl at 37°C for 1 hour for neutral sphingomyelinase (nSMase). For acid SMase (aSMase), 0.4 M sodium acetate, pH 4.5, was used instead of Tris/HCl. The reaction was stopped by the addition of 1.5 ml of chloroform/methanol (2:1 vol/vol); then 0.2 ml of doubly distilled water was added to the mixture, vortex-mixed, and centrifuged at 1,000 g for 5 minutes to separate the two phases. The clear aqueous phase was removed to a glass scintillation vial, and the radioactivity was determined by scintillation counting. The experiments were performed at least three times in duplicate.
Measurement of caspase-9 activity.
N-acetyl-Leu-Glu-His-Asp-p-nitroanilide (Ac-LEHD-pNA) cleavage was detected using a kit purchased from R&D Systems Inc. (Minneapolis, Minnesota, USA). Upon cleavage of the substrate by caspase-9, free pNA light absorbance can be quantified using a microreader plate reader at 405 nm.
Statistical analysis.
The statistical analysis was performed using an unpaired t test. P values of less than 0.05 were regarded as statistically significant.
Results
Correlation of anticancer drug–induced Cer formation and apoptosis induction with PKCδ protein expression in PC cells.
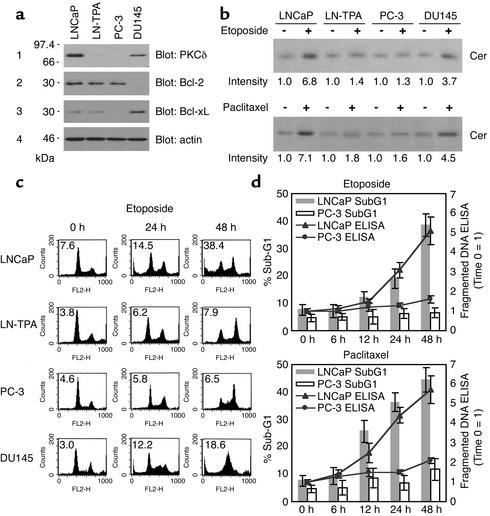
Western blot analysis revealed high levels of PKCδ proteins in total cell lysates derived from LNCaP cells, but low from LN-TPA or PC-3 cells and intermediate from DU145 cells (Figure 1a, panel 1). Bcl-2 families are known to regulate mitochondrial apoptotic process (25). Bcl-2 was expressed in LNCaP, LN-TPA, and PC-3 cells, and BCL-xL was not in PC-3 cells (Figure 1a, panels 2 and 3). Cer quantitation assay by [14C]serine labeling showed that 10 μM etoposide treatment for 24 hours resulted in 6.8- and 3.7-fold increase in Cer formation compared with untreated controls in PKCδ-positive LNCaP and DU145 cells, respectively, but not in PKCδ-negative LN-TPA or PC-3 cells (Figure 1b, upper panel). Similar results were obtained by 100 nM paclitaxel treatment (lower panel). Cell cycle analysis showed that 10-μM etoposide treatment for 0–48 hours resulted in a marked increase in sub-G1 DNA content in LNCaP and DU145 cells, but not in LN-TPA or PC-3 cells, whereas a marked increase in S or G2 DNA content was induced in all PC cell lines (Figure 1c), thus suggesting that both LN-TPA and PC-3 cells are apoptosis resistant to etoposide even though they sense etoposide-induced DNA damage. Fragmented DNA ELISA showed that treatment with 10 μM etoposide or 100 nM paclitaxel resulted in a time-dependent increase in fragmented DNA (apoptotic cells) in LNCaP cells but not in PC-3 cells, which was well correlated with the results obtained by cell cycle analysis (Figure 1d). These results suggest that anticancer drug-induced Cer formation and apoptosis both correlate well with the protein expression of PKCδ rather than Bcl-2 or Bcl-xL and that DNA damage–induced mitotic cell cycle arrest in PC cells is not associated with apoptosis induction.
Figure 1.
Correlation of anticancer drug–induced ceramide formation and apoptosis induction with PKCδ protein expression in PC cells. (a) Total cell lysates (20 μg) from PC cells were analyzed by Western blot analysis as described in Methods using specific Ab’s. (b) PC cells were treated with or without 10 μM etoposide (upper panel) or 100 nM paclitaxel (lower panel) for 24 hours, and Cer levels were determined as described in Methods. The data are expressed as fold increase relative to each control and are representative of three experiments. (c) DNA flow cytometry histograms of PC cells cultured in media containing 10% FCS treated with or without 10 μM etoposide for the indicated periods. The data are representative of three experiments. Percentage of apoptosis (sub-G1 DNA content) is indicated for each sample. (d) LNCaP and PC-3 cells were treated with 10 μM etoposide or 100 nM paclitaxel for various periods. Sub-G1 DNA content and fragmented DNA in each sample were measured by cell cycle analysis and ELISA. Bars represent SD. Experiments were repeated three times with similar results.
PKCδ activity is required for Cer formation and apoptotic cell death in response to anticancer drugs in PC cells.
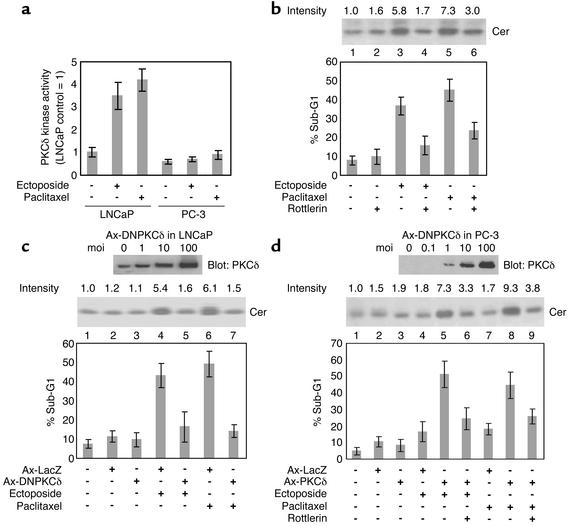
To assess whether or not PKCδ activity is needed for anticancer drug–induced Cer formation and apoptosis, we first investigated whether PKCδ could be activated by anticancer drugs. As shown in Figure 2a, incubation with 10 μM etoposide or 100 nM paclitaxel for 6 hours resulted in greater than threefold increase in PKCδ activity in LNCaP cells but not in PC-3 cells. We next cultured LNCaP cells in media containing etoposide or paclitaxel with the specific PKCδ inhibitor Rottlerin. Incubation of LNCaP cells with 10 μM etoposide or 100 nM paclitaxel for 24 hours resulted in 5.8- or 7.3-fold increase in Cer formation, respectively, and incubation for 48 hours resulted in about five- to sixfold increase in sub-G1 DNA content compared with the untreated control (Figure 2b, lanes 3 and 5, P < 0.01 compared with lane 1). Pretreatment with 10 μM Rottlerin for 2 hours reversed anticancer drug–induced Cer formation and apoptosis (lanes 4 and 6, P < 0.01). Similar results were obtained by overexpression of DNPKCδ after Ax-DNPKCδ transfection. We confirmed that DNPKCδ protein was highly expressed in LNCaP cells at concentrations of 10 moi or more 24 hours after Ax-DNPKCδ transfection (Figure 2c, upper panel). Culturing of LNCaP cells in media containing 10 μM etoposide or 100 nM paclitaxel Ax-DNPKCδ (10 moi) transfection (Figure 2c, middle panel, 24 hours, and graph, 48 hours, lanes 5 and 7) resulted in a marked decrease in Cer formation and a significant decrease in sub-G1 DNA con-tent (P < 0.01) compared with anticancer drug treat-ment after Ax-LacZ transfection (lanes 4 and 6). The anticancer drug–induced PKCδ kinase activation in LNCaP cells was significantly inhibited by Ax-DNPKCδ transfection (data not shown). We further examined whether wild-type PKCδ expression after Ax-PKCδ transfection could augment drug-induced Cer formation and apoptosis in PKCδ-negative PC-3 cells. Western blot analysis confirmed that PKCδ protein was highly expressed in PC-3 cells at concentrations of 10 moi or more 24 hours after Ax-PKCδ transfection (Figure 2d, upper panel). Culturing of PC-3 cells in media containing 10 μM etoposide or 100 nM paclitaxel following Ax-PKCδ (10 moi) transfection (Figure 2d, middle panel, 24 hours, and graph, 48 hours, lanes 5 and 8) resulted in greater than fourfold increase in Cer formation and about two- to threefold increase in sub-G1 DNA content (P < 0.01) compared with anticancer drug treatment after Ax-LacZ transfection (lanes 4 and 7). Pretreatment of PKCδ-expressing PC-3 cells with 10 μM Rottlerin (lanes 6 and 9) reversed anticancer drug–induced Cer formation and apoptosis (P < 0.01 compared with lanes 5 and 8). These results suggest that PKCδ protein expression and the kinase activity of PKCδ are required for anticancer drug–induced Cer formation and apoptosis.
Figure 2.
PKCδ activity is required for Cer formation and apoptotic cell death in response to anticancer drugs in PC cells. (a) PC cells were treated with or without 10 μM etoposide or 100 nM paclitaxel for 6 hours, and PKCδ activity was measured as described in Methods. Experiments were repeated twice with similar results. (b) LNCaP cells were treated with the complex indicated (10 μM etoposide, 100 nM paclitaxel, 10 μM Rottlerin), and Cer levels (24-hour treatment, upper panel) and apoptosis (48-hour treatment, graph) were determined. Cer data are expressed as fold increase relative to the control and are representative of three experiments. Experiments were repeated three times with similar results. LNCaP (c) and PC-3 (d) cells, infected with Ax-DNPKCδ and Ax-PKCδ at different moi’s, are incubated for 24 hours. Cell lysates were analyzed using anti-PKCδ Ab (upper panels). Note that both endogenous wild-type PKCδ and DNPKCδ were detected in LNCaP cells. LNCaP (c) and PC-3 (d) cells, infected with Ax-LacZ, Ax-DNPKCδ, or Ax-PKCδ at 10 moi, were treated with the complex indicated (10 μM etoposide, 100 nM paclitaxel, 10 μM Rottlerin), and Cer levels (middle panels) and apoptosis (graphs) were determined as described in b. For all, bars represent SD. All experiments were repeated three times with similar results.
Etoposide induces a biphasic increase in Cer formation.
It is of interest that an earlier study reported that biphasic Cer accumulation, i.e., an early and transient increase followed by a late and sustained increase in Cer, was induced by apoptotic stimuli (26). We investigated the time course of Cer formation induced by etoposide in LNCaP and PC-3 cells. Treatment of LNCaP cells with 10 μM etoposide resulted in about twofold increase in Cer levels within 1 hour, followed by a sustained increase in Cer formation beginning within 6 hours of treatment and increasing by five- to sevenfold within 36 hours (Figure 3a). In PKCδ-negative PC-3 cells, etoposide failed to induce a sustained increase in Cer formation, whereas it induced similar transient Cer increase in LNCaP cells. Cer can result from the SM cycle, and SMase has been implicated in the late Cer formation induced by etoposide as well as CD95 and γ-radiation (2, 24). We therefore investigated the role of the SM cycle and SMase in Cer formation in LNCaP cells. TLC analysis using [14C]serine labeling of the total lipids in LNCaP cells showed that etoposide treatment resulted in a marked increase in SM levels at 3 hours, followed by a gradual decrease to the basal level within 36 hours (Figure 3b). SMase assay showed that etoposide treatment for 9 hours and longer resulted in a significant increase in the nSMase activity but not in the aSMase activity (P < 0.05, Figure 3b, graph). De novo Cer generation pathway is also well-known to be rapidly activated by various anticancer drugs (26, 27). Since we have shown that PKCδ and nSMase have been implicated in etoposide-induced Cer formation in LNCaP cells, we investigated the precise role of PKCδ, nSMase, and de novo Cer synthesis in etoposide-induced biphasic Cer formation using their inhibitors, Rottlerin, 3-O-methyl-sphingomyelin (MeSM), and FB-1. As shown in Figure 3c, pretreatment of LNCaP cells with 50 μM FB-1 prior to adding 10 μM etoposide resulted in a significant decrease in Cer formation (1, 9, and 24 hours; P < 0.01). In contrast, pretreatment with 10 μM Rottlerin or 100 μM MeSM failed to decrease etoposide-induced early Cer formation (1 hour), whereas it was able to significantly inhibit the late Cer formation (9 and 24 hours; P < 0.01). These results suggest that the early Cer increase is independent of PKCδ and SM cycle and is thus derived from de novo Cer synthesis, which also has an effect on the late Cer accumulation. We next treated LNCaP cells with these inhibitors later than etoposide treatment. Treatment with Rottlerin or MeSM 3 hours later than the addition of etoposide resulted in a significant decrease in the late Cer formation (9 and 24 hours, respectively, P < 0.01), while treatment with FB-1 failed. These results suggest that the late and sustained Cer formation is, in turn, derived from SM cycle and dependent on PKCδ. We investigated whether nSMase activation was regulated by de novo Cer synthesis and PKCδ. Pretreatment with 10 μM Rottlerin or 50 μM FB-1 prior to treatment with 10 μM etoposide for 9 hours (Figure 3d, lanes 4 and 6) or treatment with Rottlerin 3 hours later than etoposide treatment (lane 8) resulted in a significant decrease in the nSMase activity (P < 0.01 compared with lane 3), while treatment with 50 μM FB-1 3 hours later than etoposide treatment (lane 7) failed. Together, these results suggest that etoposide-induced biphasic Cer increase is mediated by distinct Cer formation pathways and that PKCδ is required for the late and sustained Cer formation derived from the SM cycle.
Figure 3.
Etoposide induces a biphasic increase in Cer formation. (a) LNCaP and PC-3 cells were treated with 10 μM etoposide for various periods, and Cer levels were determined. (b) LNCaP cells were treated with 10 μM etoposide for various periods, and SM levels are determined by TLC. The nSMase and aSMase were measured as described in Methods. PE, phosphatidylethanolamine; PS, phosphatidylserine. (c) LNCaP cells were treated with the complex (10 μM etoposide, 10 μM Rottlerin, 50 μM FB-1, 100 μM MeSM) for the indicated periods, and Cer levels were determined. Etop, etoposide; Rot, Rottlerin. In each treatment, the time at which etoposide was added was set to 0 hours. (d) LNCaP cells were treated with the complex indicated (10 μM etoposide, 10 μM Rottlerin, 50 μM FB-1) for 9 hours, and nSMase activity was determined. In each treatment, the time at which etoposide was added was set to 0 hours. For all, bars represent SD. Experiments were repeated three times with similar results.
Both PKCδ and Cer are required for etoposide-induced mitochondrial events.
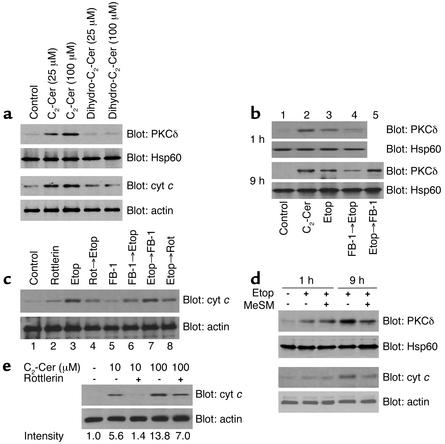
PKCδ mitochondrial translocation is important for mitochondrial events (9, 11). We also confirmed that treatment with etoposide resulted in a time-dependent increase in PKCδ mitochondrial translocation followed by cyt c release (data not shown). Recent studies suggest that Cer can also induce PKCδ translocation (14, 15), thus leading to the possibility that etoposide-induced Cer formation has an effect on PKCδ mitochondrial translocation followed by cyt c release. To clarify this possibility, we first investigated whether one of the Cer analogues C2-Cer can effect PKCδ mitochondrial translocation and cyt c release. We confirmed in a preliminary study that C2-Cer treatment resulted in a marked increase in the intracellular Cer activity (data not shown, see ref. 28). Incubation of LNCaP with C2-Cer (25–100 μM) but not biologically inactive dihydro-C2-Cer for 9 hours resulted in marked increase in PKCδ mitochondrial translocation and cyt c release (Figure 4a). We next investigated whether de novo Cer synthesis is required for etoposide-induced PKCδ mitochondrial translocation. Incubation of LNCaP with 10 μM etoposide for both 1 hour and 9 hours resulted in an increase in PKCδ mitochondrial translocation (Figure 4b, lanes 1 and 3), which was blocked by pretreatment with 50 μM FB-1 (lane 4). However, treatment with FB-1 3 hours later than the addition of etoposide (lane 5) failed to decrease etoposide-induced Cer formation in the late phase. In both phases, 25 μM C2-Cer markedly induced PKCδ mitochondrial translocation (lane 2). Similar results were obtained by Western blot analysis for cyt c release. Pretreatment with 50 μM FB-1 for 2 hours prior to etoposide treatment for 9 hours resulted in a marked decrease in cyt c release (Figure 4c, lanes 3 and 6), while treatment with FB-1 3 hours later than etoposide treatment (lane 7) failed. Recent studies suggest that PKCδ kinase activity is required for such mitochondrial events as cyt c release (9, 11). Pretreatment or treatment after administration of etoposide with 10 μM Rottlerin resulted in a marked decrease in etoposide-induced cyt c release (Figure 4c, lanes 3, 4, and 8). Our results suggest that Cer derived from de novo pathway is required but not sufficient for etoposide-induced PKCδ mitochondrial translocation followed by cyt c release in the late phase. We therefore investigated whether mitochondrial events are also mediated by the SM cycle. As shown in Figure 4d, pretreatment with 100 μM MeSM markedly blocked etoposide-induced PKCδ mitochondrial translocation and cyt c release in the late phase (9 hours) but not in the early phase (1 hour). These results suggest that Cer from de novo pathway and the SM cycle mediates etoposide-induced mitochondrial events in different phases. We further investigated whether Cer can induce cyt c release independent of PKCδ since earlier studies suggested that Cer has a direct effect on mitochondria (12, 13). Pretreatment with 10 μM Rottlerin for 2 hours prior to 10 μM C2-Cer for 9 hours resulted in over 90% decrease in cyt c release compared with C2-Cer alone, while pretreatment with Rottlerin prior to higher concentration (100 μM) of C2-Cer resulted in about 50% decrease (Figure 4e). Together, these results suggest that etoposide-induced mitochondrial events require both Cer increase and PKCδ, although the possibility remains that increased Cer can directly induce cyt c release independent of PKCδ.
Figure 4.
Both PKCδ and Cer are required for etoposide-induced mitochondrial events. (a) LNCaP cells were treated with Cer analogues at indicated concentrations for 9 hours, and cells were harvested and separated into mitochondrial and cytosol fractions. Lysates were analyzed using anti-PKCδ Ab and anti–cyt c Ab. (b) LNCaP cells were treated with the complex indicated (25 μM C2-Cer, 10 μM etoposide, 50 μM FB-1) for 1 hour or 9 hours, and mitochondrial fractions were analyzed using anti-PKCδ Ab. (c) LNCaP cells were treated with the complex indicated (10 μM etoposide,10 μM Rottlerin, 50 μM FB-1) for 9 hours, and cytosol fractions were analyzed using anti–cyt c Ab. (d) LNCaP cells were treated with the complex indicated (10 μM etoposide, 100 μM MeSM) for 1 hour or 9 hours, and mitochondrial and cytosol fractions were analyzed using anti-PKCδ Ab and anti–cyt c Ab. (e) LNCaP cells were treated with the complex indicated (C2-Cer, 10 μM Rottlerin) for 9 hours, and cytosol fractions were analyzed using anti–cyt c Ab. Intensities of cyt c bands are expressed as fold increase relative to the control.
Caspase-9 mediates the late Cer accumulation from SM cycle.
Caspase-9 processing following cyt c release is regarded as the apex of the apoptotic cascade (29). We also found that etoposide treatment for 9 hours markedly induced caspase-9 processing in LNCaP cells, which was reversed by pretreatment with 10 μM Rottlerin (Figure 5a, upper panel). Of note, treatment with etoposide and/or Rottlerin was found to have little effect on caspase-3 processing (Figure 5a, lower panel). Etoposide-induced caspase-9 activation was substantiated by a caspase-9 activity assay. Treatment with etoposide (Figure 5b, lane 3) resulted in about a fourfold increase in caspase-9 activity compared with the untreated control (lane 1, P < 0.01), which was significantly reversed by treatment with Rottlerin or pretreatment with FB-1 (lanes 4, 6, and 8; P < 0.01 compared with lane 3), but not after treatment with FB-1 (lane 7). We next investigated whether etoposide-induced sustained Cer formation resulted from caspase-9 activation, since initiator caspases such as caspase-9 can mediate DNA damage–induced Cer formation (2, 30). Pretreatment of LNCaP cells with the specific caspase-9 inhibitor LEHD-fmk or pan-caspase inhibitor zVAD-fmk resulted in a dose-dependent decrease in Cer formation induced by etoposide treatment for 24 hours, whereas pretreatment with the caspase-3 inhibitor N-acetyl-Asp-Glu-Val-aspartic acid aldehyde (DEVD-CHO) at concentrations of 20–100 μM failed to block it (Figure 5c, upper panel). Treatment of LNCaP cells with etoposide plus LEHD-fmk resulted in a significant decrease in Cer formation through the late phase (data not shown). Our findings indicate the possibility that nSMase can be activated by caspase-9. Pretreatment of LNCaP cells with LEHD-fmk or zVAD-fmk resulted in a dose-dependent decrease in nSMase activation induced by etoposide treatment for 9 hours, whereas pretreatment with DEVD-CHO failed (Figure 5c, lower graph). We finally investigated whether PKCδ mitochondrial translocation dependent on Cer is regulated by caspase-9. As shown in Figure 5d, pretreatment with 50 μM LEHD-fmk markedly inhibited 10 μM etoposide-induced PKCδ mitochondrial translocation in the late phase (9 hours, lanes 4 and 5) but not in the early phase (1 hour, lanes 2 and 3). Pretreatment with 100 μM DEVD-CHO failed to block etoposide-induced PKCδ mitochondrial translocation (lane 6). These results suggest that etoposide-induced sustained Cer accumulation and PKCδ mitochondrial translocation depends predominantly on PKCδ-mediated caspase-9 activation, which can induce sustained Cer formation via the SM cycle.
Figure 5.
Caspase-9 mediates the late Cer accumulation from the SM cycle. (a and b) LNCaP cells were cultured treated with the complex indicated (10 μM etoposide, 10 μM Rottlerin, 50 μM FB-1) for 9 hours, and cell lysates were analyzed by Western blot analysis using anti–caspase-9 Ab and anti–caspase-3 Ab (a), and caspase-9 protease activity was assayed as described in Methods (b). Bars represent SD. Experiments were repeated twice in triplicate with similar results. (c) LNCaP cells were treated with the complex indicated (10 μM etoposide, LEHD-fmk, DEVD-CHO, zVAD-fmk) for 9 hours, and Cer levels (upper panel) and SMase activity (graph) were measured. Cer data are expressed as fold increase relative to the control and are representative of three experiments. Bars represent SD. Experiments were repeated three times with similar results. (d) LNCaP cells were treated with the complex indicated (10 μM etoposide, 50 μM LEHD-fmk, 100 μM DEVD-CHO) for 1 hour or 9 hours, and mitochondrial fractions were analyzed using anti-PKCδ Ab.
Discussion
The results presented herein help clarify the findings of a previous report on anticancer drug–induced apoptotic cell death in androgen-sensitive LNCaP cells but not in androgen-independent PC cells such as PC-3 cells (31). We showed that a novel PKC isoform PKCδ protein, which was highly expressed in LNCaP cells but not in PC-3 cells, was required for apoptosis induced by paclitaxel, which targets microtubule network, as well as etoposide, which inhibits topoisomerase II. Our results are consistent with recent reports in which PKCδ mitochondrial translocation and its kinase activity have been implicated in the mitochondrial apoptotic pathway (9, 11, 32).
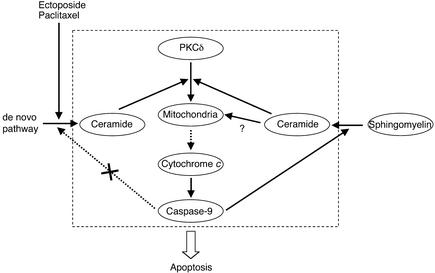
More importantly, our study demonstrated that PKCδ was required for sustained Cer formation relevant for apoptosis signaling in response to anticancer drugs (2–4). In agreement with a previous study (26), we found that etoposide induced biphasic Cer accumulation in etoposide-sensitive LNCaP cells but not in etoposide-resistant PC-3 cells. While we have shown that de novo Cer generation pathway is required for the early and transient increase in Cer, the importance of this pathway in anticancer drug–induced Cer generation and apoptosis remains controversial (5, 33). We have shown that the early and transient Cer formation is induced even in apoptosis-resistant PC-3 cells and moreover, similar to a recent study (34), we have found that serine palmitoyltransferase rather than Cer synthase is rapidly activated by etoposide treatment in LNCaP cells and even in PC-3 cells (M. Sumitomo et al., unpublished data). Our results highlight the involvement of not only de novo Cer generation pathway but the SM cycle mediated by PKCδ in anticancer drug–induced Cer accumulation in PC cells. Furthermore, our results suggest that de novo Cer synthesis, activated by PKCδ-independent mechanisms, is required for transient Cer formation followed by mitochondrial events but it is not sufficient for amplifying these events without PKCδ. In contrast, the late and sustained Cer formation, dependent on PKCδ whose mitochondrial translocation and kinase activation is followed by caspase-9 activation, is sufficient for amplifying mitochondrial events. As a result, PKCδ contributes to sustained Cer formation through caspase-9 activation in mitochondrial events. Once PKCδ translocates onto mitochondria, it can activate PKCδ-caspase-9-Cer loop independent of de novo Cer generation pathway, providing an amplifying signal to mitochondria (Figure 6).
Figure 6.
The role of PKCδ in inducing sustained Cer accumulation in mitochondrial apoptosis. Anticancer drugs induce early and transient de novo Cer synthesis. Generated Cer induces PKCδ mitochondrial translocation, which promotes cyt c release and caspase-9 activation dependent on PKCδ activity. Caspase-9 in turn induces the late and sustained Cer accumulation derived from SM by activating nSMase, which further promotes PKCδ mitochondrial translocation, thus providing an amplifying signal to mitochondria in a feedback loop of caspase activation.
It seems obvious that sustained Cer increase plays a crucial role in anticancer drug–induced apoptosis since we found that pretreatment with FB-1 or MeSM blocked etoposide-induced apoptosis in LNCaP and PKCδ-expressed PC-3 cells (data not shown). However, it remains unclear as to how increased Cer contributes to the effector phase of apoptosis. Interestingly, we found that C2-Cer induced marked apoptosis in PKCδ-negative PC-3 cells and even in LNCaP cells pretreated with LEHD-fmk (M. Sumitomo et al., unpublished data), leading to the possibility that increased Cer that resulted from the mitochondrial amplification loop needs neither PKCδ nor the downstream caspase cascade from caspase-9 in order to induce apoptosis. Tepper et al. (35) recently reported that increased Cer could directly alter the cell surface morphology, resulting in apoptosis. In addition, we found that caspase-3 processing was not recognized in etoposide-treated LNCaP cells. Fujii et al. (16) reported similar results in which TPA-induced apoptosis in LNCaP cells was mediated by PKCδ but was also independent of caspase-3. These reports and the findings of our study may provide new insight supporting the hypothesis that PKCδ-mediated apoptosis is predominantly mediated by increased Cer via mitochondrial change in an effector caspase-independent manner.
In summary, we clarified a part of the complicated mechanisms that fail to generate Cer in anticancer drug–resistant PC cells. Even in apoptosis-resistant PC cells, DNA damage and transient Cer generation could be induced, but PKCδ is essential for amplifying Cer accumulation that can further activate mitochondrial apoptosis signaling. A modification of PKCδ protein expression using neutral endopeptidase, which can block the rapid degradation of PKCδ protein (7), with anticancer drugs may thus be a potentially effective therapeutic modality for the treatment of PC cells.
Footnotes
See the related Commentary beginning on page 717.
References
- 1.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 2.Tepper AD, de Vries E, van Blitterswijk WJ, Borst J. Ordering of ceramide formation, caspase activation, and mitochondrial changes during CD95- and DNA damage-induced apoptosis. J Clin Invest. 1999;103:971–978. doi: 10.1172/JCI5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boesen-de Cock JG, et al. Common regulation of apoptosis signaling induced by CD95 and the DNA-damaging stimuli etoposide and gamma-radiation downstream from caspase-8 activation. J Biol Chem. 1999;274:14255–14261. doi: 10.1074/jbc.274.20.14255. [DOI] [PubMed] [Google Scholar]
- 4.Charles AG, et al. Taxol-induced ceramide generation and apoptosis in human breast cancer cells. Cancer Chemother Pharmacol. 2001;47:444–450. doi: 10.1007/s002800000265. [DOI] [PubMed] [Google Scholar]
- 5.Bose R, et al. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 6.Lu Z, et al. Tumor promotion by depleting cells of protein kinase C delta. Mol Cell Biol. 1997;17:3418–3428. doi: 10.1128/mcb.17.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumitomo M, et al. Neutral endopeptidase promotes phorbol ester-induced apoptosis in prostate cancer cells by inhibiting neuropeptide-induced protein kinase C delta degradation. Cancer Res. 2000;60:6590–6596. [PubMed] [Google Scholar]
- 8.Reyland ME, Anderson SM, Matassa AA, Barzen KA, Quissell DO. Protein kinase C delta is essential for etoposide-induced apoptosis in salivary gland acinar cells. J Biol Chem. 1999;274:19115–19123. doi: 10.1074/jbc.274.27.19115. [DOI] [PubMed] [Google Scholar]
- 9.Matassa AA, Carpenter L, Biden TJ, Humphries MJ, Reyland ME. PKCdelta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem. 2001;276:29719–29728. doi: 10.1074/jbc.M100273200. [DOI] [PubMed] [Google Scholar]
- 10.Dal Pra I, Whitfield JF, Chiarini A, Armato U. Increased activity of the protein kinase C-delta holoenzyme in the cytoplasmic particulate fraction precedes the activation of caspases in polyomavirus-transformed pyF111 rat fibroblasts exposed to calphostin C or topoisomerase-II inhibitors. Exp Cell Res. 2000;255:171–183. doi: 10.1006/excr.1999.4789. [DOI] [PubMed] [Google Scholar]
- 11.Majumder PK, et al. Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome crelease and apoptosis. J Biol Chem. 2000;275:21793–21796. doi: 10.1074/jbc.C000048200. [DOI] [PubMed] [Google Scholar]
- 12.Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 13.Ghafourifar P, et al. Ceramide induces cytochrome crelease from isolated mitochondria. Importance of mitochondrial redox state. J Biol Chem. 1999;274:6080–6084. doi: 10.1074/jbc.274.10.6080. [DOI] [PubMed] [Google Scholar]
- 14.Sawai H, et al. Ceramide-induced translocation of protein kinase C-delta and -epsilon to the cytosol. Implications in apoptosis. J Biol Chem. 1997;272:2452–2458. doi: 10.1074/jbc.272.4.2452. [DOI] [PubMed] [Google Scholar]
- 15.Kajimoto T, Ohmori S, Shirai Y, Sakai N, Saito N. Subtype-specific translocation of the delta subtype of protein kinase C and its activation by tyrosine phosphorylation induced by ceramide in HeLa cells. Mol Cell Biol. 2001;21:1769–1783. doi: 10.1128/MCB.21.5.1769-1783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii T, et al. Involvement of protein kinase C delta (PKCdelta) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKCdelta. J Biol Chem. 2000;275:7574–7582. doi: 10.1074/jbc.275.11.7574. [DOI] [PubMed] [Google Scholar]
- 17.Garzotto M, et al. 12-O-tetradecanoylphorbol-13-acetate-induced apoptosis in LNCaP cells is mediated through ceramide synthase. Cancer Res. 1998;58:2260–2264. [PubMed] [Google Scholar]
- 18.Powell CT, et al. Persistent membrane translocation of protein kinase C alpha during 12-O-tetradecanoylphorbol-13-acetate-induced apoptosis of LNCaP human prostate cancer cells. Cell Growth Differ. 1996;7:419–428. [PubMed] [Google Scholar]
- 19.Sumitomo M, et al. Neutral endopeptidase inhibits prostate cancer cell migration by blocking focal adhesion kinase signaling. J Clin Invest. 2000;106:1399–1407. doi: 10.1172/JCI10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohba M, et al. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol Cell Biol. 1998;18:5199–5207. doi: 10.1128/mcb.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumitomo M, et al. Induction of apoptosis of cytokine-producing bladder cancer cells by adenovirus-mediated IkappaBalpha overexpression. Hum Gene Ther. 1999;10:37–47. doi: 10.1089/10430349950019174. [DOI] [PubMed] [Google Scholar]
- 22.Kuroki T, Kashiwagi M, Ishino K, Huh N, Ohba M. Adenovirus-mediated gene transfer to keratinocytes—a review. J Investig Dermatol Symp Proc. 1999;4:153–157. doi: 10.1038/sj.jidsp.5640200. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Yu JC, Shin DY, Pierce JH. Characterization of a protein kinase C-delta (PKC-delta) ATP binding mutant. An inactive enzyme that competitively inhibits wild type PKC-delta enzymatic activity. J Biol Chem. 1995;270:8311–8318. doi: 10.1074/jbc.270.14.8311. [DOI] [PubMed] [Google Scholar]
- 24.Tepper AD, Diks SH, van Blitterswijk WJ, Borst J. Glucosylceramide synthase does not attenuate the ceramide pool accumulating during apoptosis induced by CD95 or anti-cancer regimens. J Biol Chem. 2000;275:34810–34817. doi: 10.1074/jbc.M005142200. [DOI] [PubMed] [Google Scholar]
- 25.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 26.Cuvillier O, Edsall L, Spiegel S. Involvement of sphingosine in mitochondria-dependent Fas-induced apoptosis of type II Jurkat T cells. J Biol Chem. 2000;275:15691–15700. doi: 10.1074/jbc.M000280200. [DOI] [PubMed] [Google Scholar]
- 27.Senchenkov A, Litvak DA, Cabot MC. Targeting ceramide metabolism—a strategy for overcoming drug resistance. J Natl Cancer Inst. 2001;93:347–357. doi: 10.1093/jnci/93.5.347. [DOI] [PubMed] [Google Scholar]
- 28.Abe A, Shayman JA, Radin NS. A novel enzyme that catalyzes the esterification of N-acetylsphingosine. Metabolism of C2-ceramides. J Biol Chem. 1996;271:14383–14389. doi: 10.1074/jbc.271.24.14383. [DOI] [PubMed] [Google Scholar]
- 29.Li P, et al. Cytochrome cand dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 30.Grullich C, Sullards MC, Fuks Z, Merrill AH, Jr, Kolesnick R. CD95(Fas/APO-1) signals ceramide generation independent of the effector stage of apoptosis. J Biol Chem. 2000;275:8650–8656. doi: 10.1074/jbc.275.12.8650. [DOI] [PubMed] [Google Scholar]
- 31.Wang XZ, Beebe JR, Pwiti L, Bielawska A, Smyth MJ. Aberrant sphingolipid signaling is involved in the resistance of prostate cancer cell lines to chemotherapy. Cancer Res. 1999;59:5842–5848. [PubMed] [Google Scholar]
- 32.Li L, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH. Protein kinase Cdelta targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol Cell Biol. 1999;19:8547–8558. doi: 10.1128/mcb.19.12.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaffrezou JP, et al. Daunorubicin-induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J. 1996;15:2417–2424. [PMC free article] [PubMed] [Google Scholar]
- 34.Perry DK, et al. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J Biol Chem. 2000;275:9078–9084. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- 35.Tepper AD, et al. Sphingomyelin hydrolysis to ceramide during the execution phase of apoptosis results from phospholipid scrambling and alters cell-surface morphology. J Cell Biol. 2000;150:155–164. doi: 10.1083/jcb.150.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]