Abstract
To date, most studies have focused on the characterization of HIV-1–specific cellular immune responses in the peripheral blood (PB) of infected individuals. Much less is known about the comparative magnitude and breadth of responses in the lymphoid tissue. This study analyzed HIV-1–specific CD8+ T cell responses simultaneously in PB and lymph nodes (LNs) of persons with chronic HIV-1 infection and assessed the dynamics of these responses during antiretroviral treatment and supervised treatment interruption (STI). In untreated chronic infection, the magnitude of epitope-specific CD8+ T cell activity was significantly higher in LNs than in PB. Responses decreased in both compartments during highly active antiretroviral therapy, but this decline was more pronounced in PB. During STI, HIV-1–specific CD8+ T cell responses in PB increased significantly. Enhancement in breadth and magnitude was largely due to the expansion of pre-existing responses in the LNs, with new epitopes infrequently targeted. Taken together, these data demonstrate that HIV-1–specific CD8+ T cells are preferentially located in the LNs, with a subset of responses exclusively detectable in this compartment. Furthermore, the enhanced CD8+ T cell responses observed during STI in chronically infected individuals is largely due to expansion of pre-existing virus-specific CD8+ T cells, rather than the induction of novel responses.
Introduction
Since the first clinical evidence of AIDS was reported two decades ago, HIV-1 has killed over 20 million people worldwide, and an estimated 40 million people are infected with HIV-1. The development of highly active antiretroviral therapy (HAART) has led to substantial advances in the management of HIV-1–infected individuals. However, this therapy is limited due to resistance development, toxicity, high costs, and difficulties with delivery in resource-poor areas. The majority of infected individuals who live in sub-Saharan Africa and Southeast Asia have no access to antiretroviral treatment. Alternative therapeutic approaches, such as immunization or immunotherapeutic interventions, are therefore needed to control the rapidly spreading HIV-1 epidemic worldwide.
The basis for the rational development of immunotherapeutic interventions is a detailed understanding of HIV-1 pathogenesis and the correlates of protective immunity. Accumulating data have shown a central role for HIV-1–specific cytotoxic T lymphocytes (CTLs) and T helper cells in controlling viral replication (1–8). Studies in the SIV/macaque model provide compelling data in support of this, showing that removal of CD8+ T cells (and therefore CTLs) in vivo results in a rapid increase in the steady-state level of SIV viremia (9, 10). Recent studies using supervised treatment interruption (STI) as an immunotherapeutic intervention have shown that HIV-1–specific cellular immune responses can be enhanced in infected individuals (11–17). In individuals treated during acute HIV-1 infection, this enhancement of HIV-1–specific CTL responses was associated with at least transient immune control of viremia (12). All these studies have focused on the characterization of HIV-1–specific immune responses in the peripheral blood (PB) of infected individuals.
Much less is known about the role of virus-specific cellular immune responses in the lymphoid tissues, which harbor more than 95% of the body’s lymphocytes and represent the major sites of virus replication and primary and secondary immune responses (18). In HIV-1–infected individuals, massive infiltration of secondary lymphoid organs with activated CD8+ T cells has been reported (19–23). HIV-1–specific CD8+ T cells colocalize with HIV-1–producing cells in the lymphoid tissue and may mediate antiviral activity (24–26). However, neither the relationship between virus-specific CTL responses in the lymphoid tissue and the PB in untreated infection, nor the dynamics of CTL responses in these two compartments during immunotherapeutic interventions, is known.
In this study, we assessed virus-specific CD8+ T cell responses in the PB and lymph nodes (LNs) of HIV-1–infected individuals at the single-epitope level. Responses in these two compartments were compared during untreated chronic infection, and the impact of antiretroviral treatment and STI on these CD8+ T cell responses was determined in longitudinal studies.
Methods
Study subjects.
Fifteen asymptomatic, chronically HIV-1–infected Caucasian males who were naive to antiretroviral therapy were enrolled in this study. For all studied individuals, matched PBMCs and LNMCs from the same timepoint were available for analysis prior to initiation of highly active antiretroviral treatment (HAART). At the time of the first analysis of HIV-1–specific immune responses, mean viral load was 318,767 ± 685,699 copies HIV-1 RNA/ml, and mean CD4+ T cell counts were 562 cells/μl in the study subjects. Second LNMC and PBMC samples were available from five of the 15 individuals after 12 months of treatment with HAART. All patients had undetectable viremia (less than 25 HIV-1 RNA copies/ml) at the time of the second LN biopsy. In situ hybridization revealed no or minimal residual HIV-1 RNA in LNs (27). The mean CD4+ T cell count at that time had increased from 562 cells/μl before therapy to 931 cells/μl. Six of the 15 study subjects, including the five individuals who gave a second LNMC sample, underwent a single STI. All six experienced viral load rebound within 1.5 to 6 weeks (median 3 weeks) to pretreatment levels (447,438 ± 910,960 copies HIV-1 RNA/ml before treatment vs. 227,750 ± 366,923 copies/ml after STI, P = 0.54). A third PBMC sample from these individuals was available for analysis at peak viremia after STI (median 4 weeks). HLA class I typing was performed using flow cytometric analyses after staining with the corresponding mAb.
Preparation of cells.
Axillary LN biopsies were performed under local anesthesia after written informed consent. LN samples were placed in normal saline at 4°C and immediately transferred to the laboratory for further processing. Mononuclear cells were mechanically liberated from lymphoid tissue, and LNMCs were frozen in FCS plus 10% DMSO. Fresh PBMCs were separated from PB by density gradient centrifugation using Ficoll-Hypaque (Sigma Chemical Co., St. Louis, Missouri, USA) and frozen in FCS plus 10% DMSO.
Synthetic HIV-1 peptides.
Peptides corresponding to described optimal HIV-1 clade B CTL epitopes (28) were synthesized on an automated peptide synthesizer (model 396 MBS and Omega; Advance ChemTech, Louisville, Kentucky, USA). A total of 107 different optimal CTL epitopes described for the HLA class I alleles expressed in the study cohort were tested, including epitopes contained within all HIV-1 proteins except HIV-1 Vpu. Each individual was tested for responses directed against all optimal CTL epitopes described for his corresponding HLA class I type (28).
ELISpot assay.
HIV-1–specific CD8+ T-cell responses were quantified by ELISpot assay as previously described (29, 30). In brief, cells were thawed and incubated for 8–10 hours in R10 medium (RPMI 1640 medium plus 10% FCS) at 37°C in 5% CO2. After counting, viable cells were plated out at 50,000 to 100,000 PBMCs or LNMCs per well, with peptides at a final concentration of 10–5 M in 96-well polyvinylidene difluoride-backed plates (MAIP S45; Millipore Corp., Bedford, Massachusetts, USA). Plates were precoated with 0.5 μg/ml anti–IFN-γ mAb (1-DIK; Mabtech AB, Stockholm, Sweden) overnight at 4°C. For negative controls, 100,000 PBMCs and LNMCs were incubated with R10 alone. The plates were incubated overnight (14–16 hours) at 37°C in 5% CO2 and washed. Next, biotinylated anti–IFN-γ mAb 7-B6-1 (Mabtech AB) was added at 0.5 μg/ml and incubated at room temperature for 90 minutes. After washing, 100 μl of streptavidin-conjugated alkaline phosphatase (diluted 1:2,000; Mabtech AB) was added per well, and incubation continued at room temperature for 45 minutes. Individual IFN-γ–producing cells were detected as dark spots after a 20- to 30-minute color reaction with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (Bio-Rad Laboratories, Hercules, California, USA). The number of specific IFN-γ–secreting T cells was counted by direct visualization, calculated by subtracting the negative-control value, and expressed as spot-forming cells (SFCs) per million input cells. Negative controls always had less than 30 SFCs per million input cells. In addition, for each studied sample, the percentage of CD4+ and CD8+ T cells was determined by flow cytometry, and magnitudes of responses were normalized to SFCs per million CD8+ T cells.
Flow-cytometric detection of antigen-induced intracellular IFN-γ secretion.
Intracellular cytokine staining assays were performed as described previously, with minor modifications (7, 31, 32). Briefly, 0.5 × 106 to 1 × 106 PBMCs or LNMCs were incubated with 4 μM peptide and 1 μg/ml each of the anti-CD28 and anti-CD49d mAb’s (Becton Dickinson Immunocytometry Systems, San Jose, California, USA) at 37°C in 5% CO2 for 1 hour, before the addition of 10 μg/ml of brefeldin A (Sigma Chemical Co.). Following a further 6-hour incubation at 37°C in 5% CO2, the cells were placed at 4°C overnight. Cells were then washed and stained with the surface Ab’s anti–CD8-PerCP and anti–CD4-APC (Becton Dickinson Immunocytometry Systems) at 4°C for 30 minutes. After washing, the cells were fixed and permeabilized using the Fixation/Permeabilization Kit from Caltag Laboratories Inc. (Burlingame, California, USA) for 15 minutes at room temperature in the dark. FITC-conjugated anti–IFN-γ was added (Becton Dickinson Immunocytometry Systems), and incubation continued at 4°C for 30 minutes. Cells were then washed and analyzed on a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems). Control conditions were established by the use of autologous PBMCs or LNMCs, which were treated identically but without peptide stimulation.
Statistical analysis.
Statistical analysis and graphical presentation was done using SigmaPlot 5.0 (SPSS Inc., Chicago, Illinois, USA). Results are given as mean ± SD or median with range. Statistical analysis of significance (P values) was based on the two-tailed Student t test and Spearman’s rank correlation.
Results
HIV-1–specific CD8+ T cell responses in PB and LNs in untreated individuals with chronic HIV-1 infection.
In order to address potential differences in HIV-1–specific CD8+ T cell responses between PB and LNs, we characterized responses in these two compartments from identical timepoints in 15 chronically HIV-1–infected individuals prior to the initiation of treatment. Responses to a median of 19 CTL epitopes described for the respective HLA class I types were tested per individual (range 5–32). In each case, the same epitopes were tested using LNMCs and PBMCs.
HIV-1–specific CD8+ T cell responses were detectable in all 15 individuals in PBMCs and LNMCs. Magnitudes of total HIV-1–specific CD8+ T cell responses were similar in PBMCs and LNMCs (1,020 ± 1,011 SFCs/million PBMCs compared with 1,114 ± 756 SFCs/million LNMCs, P = 0.8). As the percentages of CD8+ T cells were significantly lower in the LN samples than in the PB samples (38.1% ± 11.9% CD8+ LNMCs vs. 50.3% ± 9.5% CD8+ PBMCs, P < 0.01), the normalized magnitude of total HIV-1–specific responses per million CD8+ T cells was significantly higher in LNs than in PB (3,293 ± 2,467 SFCs/million CD8+ LNMCs vs. 1,938 ± 1,766 SFCs/million CD8+ PBMCs, P < 0.01).
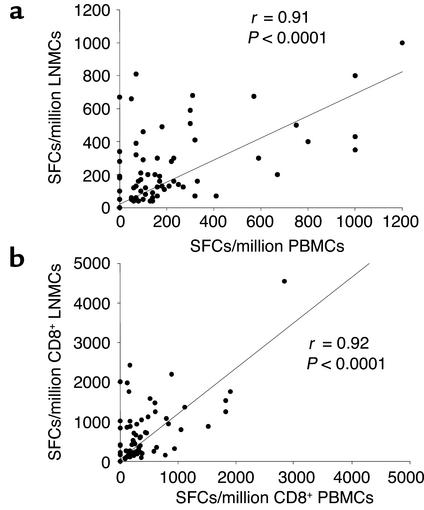
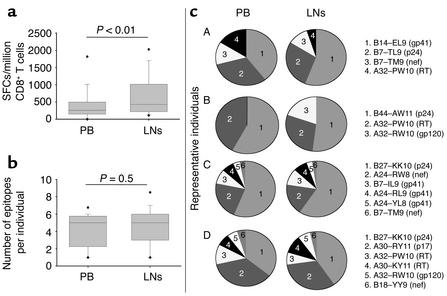
At the single CTL epitope level, HIV-1–specific CD8+ T cells in PB correlated significantly to responses in LNs, whether analyzed per number of total input cells (Figure 1a) or per number of input CD8+ T cells (Figure 1b). These correlations remained significant when limited to the 68 epitopes recognized in both LN and PB samples (r = 0.38, P = 0.0014 for total input cells; r = 0.38, P = 0.0015 after normalization to input CD8+ T cells). As described for total HIV-1–specific CTL responses, the magnitude of epitope-specific responses per million CD8+ T cells was significantly higher in LNs than in PB (Figure 2a), but the breadth of responses was similar in both compartments (Figure 2b). An average of four optimal CTL epitopes (median 5, range 1–7) per individual was targeted in the PB, compared with an average of five epitopes (median 5, range 1–9) in the LNs (P = 0.5). The hierarchy in magnitude of responses to individual CTL epitopes within a single individual was similar in LNs and PB, as shown for four representative individuals in Figure 2c. However, of the 15 persons studied, five had detectable responses in the LN specimen that were undetectable in the PB sample. Another way to express this is that of the 68 epitope-specific responses detectable in the entire cohort, CD8+ T cell responses to a total of eight epitopes were detectable only in the LN specimen, but not in PB, as shown in Figure 1 and for individual B in Figure 2c. In contrast, all responses detected in the PB were also detected in the LN samples.
Figure 1.
Correlation between CD8+ T cell responses to optimal HIV-1 CTL epitopes in the LNs and the PB. (a) Responses to each epitope in LNMCs and PBMCs are represented by a single dot. Frequencies are given as SFCs per million input PBMCs or LNMCs. (b) Frequencies after adaptation to the percentage of CD8+ T cells in each sample. Statistical analysis was performed using Spearman’s rank correlation.
Figure 2.
(a) Magnitude of epitope-specific CD8+ T cell responses in PB and LNs. Magnitudes of responses in each compartment are given as SFCs/million CD8+ T cells, and presented as box plots showing the median and the 5th/95th percentile. Statistical significance of differences between the magnitude of responses to individual CTL epitopes in PB and LNs was calculated by two-tailed Student t test. (b) Breadth of epitope-specific CD8+ T cell responses in PB and LNs. Breadth of CD8+ T cell responses in the 15 individuals studied is presented as a box plot for each compartment, showing the median and the 5th/95th percentile. Statistical significance of differences between the breadth of responses in PB and LNs was calculated by two-tailed Student t test. (c) Contribution and hierarchy of individual HIV-1–specific CTL epitopes to the total virus-specific response in PB and LNs in four representative individuals (A–D). The individual epitopes recognized are specified in the legend and described by the restricting HLA class I allele, the first and last amino acids, the length of the peptide, and the HIV-1 protein.
In a small subset of individuals (n = 3) in whom sufficient cells were available from PB and LNs, direct comparison of epitope-specific IFN-γ production of CD8+ T cells by intracellular cytokine staining also showed stronger CD8+ T cell responses in the LNMCs than in PBMCs (data not shown), supporting the initial findings of stronger HIV-1–specific CD8+ T cell responses in the LNs. No significant correlation between the magnitude or breadth of HIV-1–specific CD8+ T cell responses and HIV-1 viral load was observed in the LNs or PB.
Taken together, these data indicate that the results of analysis of HIV-1–specific CD8+ T cells in the PB largely mirror the responses in the LNs. However, virus-specific CD8+ T cells appear to be enriched in the LNs, a major site of viral replication.
Impact of antiretroviral treatment on the dynamics of HIV-1–specific CD8+ T cell responses in LNs and PB.
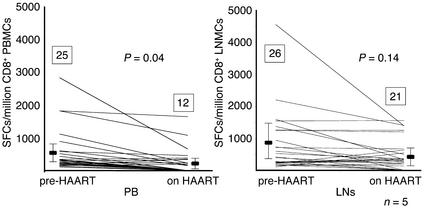
We next evaluated the impact of HAART on the magnitude and breadth of HIV-1–specific CD8+ T cell responses in LNs and PB. This analysis was performed on five of the 15 individuals described above for whom second LNMC and PBMC samples were available after 1 year of effective treatment with HAART. In all study subjects, plasma viral load was suppressed to below the level of detection (25 copies HIV-1 RNA/ml) on HAART. Treatment with HAART reduced HIV-1–specific CD8+ T cell responses in both compartments, but the decline in responses to individual CD8+ T cell epitopes was more pronounced in the PB (from 550 ± 661 to 227 ± 387 SFCs/million CD8+ PBMCs, P = 0.04) than in the LNs (from 792 ± 949 to 479 ± 476 SFCs/million CD8+ LNMCs, P = 0.14) (Figure 3). In addition, the number of detectable CTL epitopes declined in the PBMCs from an average of five epitopes per individual (median 5, range 3–6) to an average of two epitopes (median 1, range 1–6) after 1 year of treatment with HAART, representing a total loss of 13 of 25 (52%) previously detectable CD8+ T cell responses in the five individuals studied (Figure 3a). In contrast, only five of 26 (19%) responses to previously detectable CTL epitopes were lost in the LNs on treatment, reducing the average number of epitopes targeted per individual from five (median 6, range 3–6) to four (median 5, range 2–6) (Figure 3b). The positive correlation between HIV-1–specific CD8+ T cell responses in PBMCs and LNMCs was maintained during treatment (r = 0.72, P < 0.0001 for total input cells and r = 0.72, P < 0.0001 after normalization for input CD8+ T cells).
Figure 3.
Decline of HIV-1–specific CD8+ T cell responses to individual CTL epitopes upon treatment with HAART in the five individuals studied in the PB (left) and LNs (right). Epitope-specific CD8+ T cell responses are shown prior to the initiation of HAART (pre-HAART) and after 12 months on HAART (on HAART). Magnitudes of responses are given as SFCs/million CD8+ T cells, and each line demonstrates the evolution of responses in an individual CTL epitope. Mean magnitude of HIV-1–specific CD8+ T cell responses ± SD are shown before HAART and after 12 months on HAART, as is the total number of recognized CTL epitopes (numbers inside boxes) in the five individuals. Statistical significance of differences between the magnitude of responses to individual CTL epitopes before HAART and after 12 months on HAART were calculated by two-tailed Student t test.
Taken together, these studies demonstrate that a subset of epitope-specific CD8+ T cell responses is detectable in the LNs, but not in the PB. This was observed during chronic HIV-1 infection, prior to the initiation of treatment, for a total of eight of 68 detectable epitopes (12%) in the 15 individuals studied, and was even more pronounced (eight of 21 epitopes, or 38%) in the five individuals studied after 1 year of treatment. These data indicate that LNs may serve as a reservoir for virus-specific CD8+ T cell responses when antigen load is effectively suppressed during treatment with HAART.
Expansion of HIV-1–specific CD8+ T cell responses during STI.
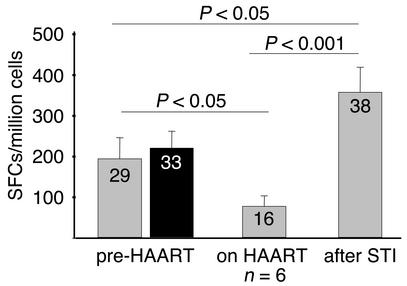
Recent studies have demonstrated the enhancement of HIV-1–specific CD8+ T cell responses in individuals who underwent STI (11–17). However, it is not fully understood whether this enhancement of responses is due to the expansion of pre-existing responses to levels that allow their detection using ex vivo assays or whether it represents the priming of novel specificities. In order to address this question, we followed six individuals from this cohort (including the five individuals described above who gave a second LN sample after 1 year of treatment, and one additional individual for whom only a baseline LN sample was available) longitudinally during a single STI. All six of these chronically infected individuals experienced a rebound in viremia, within a median of 3 weeks after interruption of treatment, to pretreatment values (447,438 ± 910,960 copies HIV-1 RNA/ml before treatment vs. 227,750 ± 366,923 copies/ml after STI, P = 0.54). The re-exposure to antigen was associated with an enhancement of HIV-1–specific CD8+ T cell responses in all six individuals. HIV-1–specific CD8+ T cell response to individual CTL epitopes increased significantly (P = 0.001) compared with CD8+ T cell responses after 1 year of treatment (Figure 4), and reached even higher levels than prior to initiation of treatment (P < 0.05). This enhancement in the magnitude of HIV-1–specific CD8+ T cell responses was associated with the development of responses to 22 epitopes in the six individuals studied that were not detectable while on therapy (median 3.5 novel epitopes per individual, range 1–6 epitopes) (Figure 4). These responses to newly targeted epitopes included responses to 13 epitopes recognized in the PB at baseline, prior to the initiation of HAART, as well as responses to nine new epitopes (median 1 epitope per individual, range 0–4) that were detectable for the first time in the PB after STI (Figure 4).
Figure 4.
Mean epitope-specific CD8+ T cell frequencies plus SD in the PB (gray bars) and LNs (black bar) prior to initiation of HAART, as well as mean epitope-specific CD8+ T cell frequencies plus SD in the PB(gray bars) after 12 months on HAART and at the time of peak viremia after STI in the six individuals studied. Mean magnitudes of epitope-specific responses are given as SFCs/million cells. The total number of targeted epitopes in the six individuals is shown in each box. Statistical significance of differences between the magnitude of responses to individual CTL epitopes was calculated by two-tailed Student t test.
Interestingly, four of these nine newly targeted epitopes were already detectable in the LN specimen at the time of initial treatment (Figure 4). Additionally, in the five individuals for whom a second LN and PB sample was available after 1 year of treatment with HAART, all the responses to epitopes that were lost during HAART in the PB, but persisted in the LNs at that time (n = 8/21, Figure 3), became detectable again in the PB after treatment interruption.
Taken together, these data demonstrate that the enhancement of HIV-1–specific CD8+ T cell responses during STIs is largely the result of the expansion of pre-existing responses. Epitope-specific CD8+ T cells may persist in the lymphoid tissue during antiretroviral treatment, even if these specific CD8+ T cells are not detectable in the PB using the same methods. After re-exposure to antigen, these responses are among the first to be detectable in the PB, and are largely responsible for the enhancement of CD8+ T cell responses observed during STI.
Discussion
Most human studies of virus-specific cellular immune responses have been performed on lymphocytes derived from the PB, and very little is known about the relative distribution of epitope-specific CD8+ T cells directed against HIV-1 in the PB compared with the CD8+ T cells directed against HIV-1 in the secondary lymphoid tissues. Here we use a sensitive IFN-γ ELISpot assay and a large panel of peptides corresponding to optimal CTL epitopes to examine the magnitude and specificity of HIV-1–specific CD8+ T cells in the PB and axillary LNs of 15 individuals with untreated chronic HIV-1 infection. In addition, the dynamics of epitope-specific CD8+ T cell responses were assessed longitudinally in a subset of individuals following the initiation of antiretroviral treatment, as well as during STI. We demonstrate that HIV-1–specific CD8+ T cells are enriched in the LNs in chronic infection, with a subset of responses that are detectable exclusively in this compartment. The preferential location of virus-specific CD8+ T cells in LNs was most pronounced during effective antiretroviral therapy, when there is a relative absence of antigen in the PB. Moreover, new HIV-1–specific CD8+ T cells that arose in the PB during treatment cessation in chronic infection largely reflect antigen-specific CD8+ T cells that persist in LNs, rather than the induction of novel responses.
LNs represent one of the main reservoirs for HIV-1, and substantially more HIV-1 replication takes place in the LNs than in the PB in infected individuals (18, 33, 34). Within lymphoid organs, massive infiltration of clonally expanded CD8+ T cells has been observed (19–23, 26, 35). In this study we used a large panel of described optimal HIV-1–specific CTL epitopes to characterize virus-specific CD8+ T cell responses. Even if this approach may underestimate total HIV-1–specific CD8+ T cell responses compared with the assess-ment of responses directed against the entire expressed HIV-1 genome (30, 36, 37), our data show polyclonal responses to multiple HIV-1 epitopes in both compartments. The magnitude and specificity of CD8+ T cell responses in the PB correlated significantly with responses detected in LNs from the same timepoint in the untreated chronically infected individuals studied. Moreover, the hierarchy in immunodominance of these responses was similar in both compartments. The magnitude of CD8+ T cell responses was found to be significantly higher in the LNs, and in every case where differences in the breadth of responses were detected, the broader response was seen in the LNs. Of the 15 subjects studied, five had more epitopes targeted by CD8+ T cells in the LNs than in the PB. In contrast, in none of the persons studied was an epitope targeted exclusively in the PB. For those epitopes targeted in the LNs and not in the PB, most responses were of low magnitude, such that it is possible that the responses were present in the PB but were just below the limit of detection. Nevertheless, our data show that when using the sensitive IFN-γ ELISpot assay, LNs provide a better reflection of the breadth of immune responses during chronic infection than does the PB.
HAART has been shown to reduce HIV-1–specific CD8+ T cell responses in the PB (38, 39). This is presumably due to the suppression of viral antigen in the PB to undetectable levels. We evaluated the dynamics of HIV-1–specific CD8+ T cell responses after the initiation of HAART in five subjects for whom a second LN sample was available after 12 months of treatment. Magnitude and breadth of HIV-1–specific CD8+ T cell responses declined in both compartments, but the decline in responses was more pronounced in the PB. In this compartment, virus-specific CD8+ T cell responses to more than 50% of the previously recognized CTL epitopes were lost during the first 12 months of treatment. In contrast, the decline of virus-specific CD8+ T cell responses in the LNs did not reach statistical significance, and responses to only five of 26 targeted CTL epitopes (19%) decreased to below the level of detection in the five individuals studied. Despite these declines in some epitope-specific responses, HIV-1–specific CD8+ T cell responses did not become entirely undetectable after 12 months on HAART in any of the subjects. Thus, the more sensitive IFN-γ ELISpot assay indicates persistence of memory CD8+ T cell responses to HIV-1 even when viremia is undetectable for prolonged periods. This observation is in line with recent reports on detectable virus-specific CD8+ T cell responses in the lymphoid tissue following treated acute HIV-1 infection (40), and with murine studies showing persistence of CTL responses in the absence of detectable viral antigen (41).
The preferential persistence of HIV-1–specific CD8+ T cells in the LNs compared with the PB of chronically infected individuals on HAART may be explained by several factors. It has been shown that residual replication of HIV-1 can persist in the LNs during HAART even in the absence of detectable virus in the PB (27, 42). The findings that some responses disappear and others persist suggest that there may be differences in the antigenic threshold for different epitope-specific responses. It is also possible that persistence of antigen rather than ongoing replication in the lymphoid tissue may lead to a persistent activation of virus-specific CD8+ T cells in the LNs, preventing a decline of these responses to a memory level below the detection limit of currently used assays. Alternatively, virus-specific CD8+ T cells may have homed from the PB to the lymphoid organs after suppression of viral replication. Taken together, these data indicate that the LNs may serve as a reservoir for virus-specific CD8+ T cells in the absence of detectable antigen in the PB during HAART.
Previous studies have shown that re-exposure to virus following treatment cessation in chronic infection leads to increases in CTL responses (11, 13, 15, 17), but whether this leads to induction of responses to novel epitopes or reflects an augmentation of pre-existing responses is not clear. In all six individuals studied, rebound in HIV-1 viremia was associated with a significant enhancement in breadth and magnitude of HIV-1–specific CD8+ T cell responses compared with levels measured during HAART. This enhancement was largely due to an enhancement of responses that were previously detectable in the LNs, but were absent or lost in the PB during treatment. Thus the enhancement of HIV-1–specific CD8+ T cell responses during STI may be largely due to the expansion of pre-existing responses from the lymphoid tissue, rather than to the induction of new responses. Given that the rebounding virus may already have escaped from effective immune control, the relative infrequency of induction of new responses represents a challenge for augmenting immune control through STI in chronic infection. This is in line with recent studies suggesting that the majority of antigen-specific CD8+ T cell responses are primed during the initial phase of infection (43–45), and suggests that alternative measures such as therapeutic immunization should be evaluated to determine if new specificities can be reliably induced.
In conclusion, this study demonstrates that HIV-1–specific CD8+ T cells are preferentially located in the LNs (compared with PB) including a subset of responses that is detectable exclusively in the secondary lymphoid tissue. Expansion of HIV-1–specific CD8+ T cells pre-existing in the LNs contributes importantly to the enhancement of virus-specific CTL responses observed in the PB of chronically infected individuals when treatment is discontinued. The simple expansion of pre-existing CD8+ T cell responses that failed to control viral replication prior to the initiation of antiretroviral treatment may have contributed to the limited success of STI in these individuals with chronic HIV-1 infection. This emphasizes the need for more potent immunotherapeutic interventions to induce antiviral immunity in chronically infected individuals to levels that may allow for immune control of HIV-1 replication.
Acknowledgments
This study was supported by the Doris Duke Charitable Foundation (to M. Altfeld and B.D. Walker), the NIH (grants R37 AI-128568, R01 AI-30914, R01 AI-44656, and R01 AI-40873), the Foundation for AIDS & Immune Research, Los Angeles, California, USA (M. Altfeld), and the Federal Ministry of Research, Germany (grant 01Ki9717/4; J. van Lunzen, N. Frahm, and H.-J. Stellbrink). The continuous support of Christiane Moecklinghoff (Hoffman LaRoche Ltd., Grenzach, Germany) to J. van Lunzen and H.-J. Stellbrink is gratefully acknowledged. B.D. Walker is the recipient of a Doris Duke Distinguished Clinical Scientist Award.
Footnotes
Marcus Altfeld and Jan van Lunzen contributed equally to this work.
References
- 1.Klein MR, et al. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker BD, et al. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987;328:345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- 3.Ogg GS, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koup RA, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immu-nodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg ES, et al. Vigorous HIV-1–specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 7.Pitcher CJ, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 8.McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–987. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz JE, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 10.Jin X, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz L, et al. HIV dynamics and T-cell immunity after three structured treatment interruptions in chronic HIV-1 infection. AIDS. 2001;15:F19–F27. doi: 10.1097/00002030-200106150-00001. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg ES, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz GM, et al. HIV-1–specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Invest. 1999;104:R13–R18. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lisziewicz J, et al. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med. 1999;340:1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 15.Garcia F, et al. The virological and immunological consequences of structured treatment interruptions in chronic HIV-1 infection. AIDS. 2001;15:F29–F40. doi: 10.1097/00002030-200106150-00002. [DOI] [PubMed] [Google Scholar]
- 16.Altfeld M, Walker BD. Less is more? STI in acute and chronic HIV-1 infection. Nat Med. 2001;7:881–884. doi: 10.1038/90901. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz GM, et al. Structured antiretroviral treatment interruptions in chronically HIV-1- infected subjects. Proc Natl Acad Sci USA. 2001;98:13288–13293. doi: 10.1073/pnas.221452198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stellbrink HJ, van Lunzen J. Lymph nodes during antiretroviral therapy. Curr Opin Infect Dis. 2001;14:17–22. doi: 10.1097/00001432-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Racz P, et al. Lymphatic tissue changes in AIDS and other retrovirus infections: tools and insights. Lymphology. 1990;23:85–91. [PubMed] [Google Scholar]
- 20.Tenner-Racz K, et al. Cytotoxic effector cell granules recognized by the monoclonal antibody TIA-1 are present in CD8+ lymphocytes in lymph nodes of human immunodeficiency virus-1-infected patients. Am J Pathol. 1993;142:1750–1758. [PMC free article] [PubMed] [Google Scholar]
- 21.Tenner-Racz K, et al. The unenlarged lymph nodes of HIV-1–infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J Exp Med. 1998;187:949–959. doi: 10.1084/jem.187.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheynier R, et al. HIV and T cell expansion in splenic white pulps is accompanied by infiltration of HIV-specific cytotoxic T lymphocytes. Cell. 1994;78:373–387. doi: 10.1016/0092-8674(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda MJ, et al. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and cell-associated viral RNA levels in distinct lymphoid compartments of SIVmac- infected rhesus monkeys. Blood. 2000;96:1474–1479. [PubMed] [Google Scholar]
- 24.Brodie SJ, et al. HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death. J Clin Invest. 2000;105:1407–1417. doi: 10.1172/JCI8707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Brodie SJ, et al. In vivo migration and function of transferred HIV-1–specific cytotoxic T cells. Nat Med. 1999;5:34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 26.Hosmalin A, et al. HIV-specific effector cytotoxic T lymphocytes and HIV-producing cells colocalize in white pulps and germinal centers from infected patients. Blood. 2001;97:2695–2701. doi: 10.1182/blood.v97.9.2695. [DOI] [PubMed] [Google Scholar]
- 27.Stellbrink HJ, et al. Kinetics of productive and latent HIV infection in lymphatic tissue and peripheral blood during triple-drug combination therapy with or without additional interleukin-2. Antivir Ther. 1998;3:209–214. [PubMed] [Google Scholar]
- 28.Brander, C., and Goulder, P.J.R. 1999. Recent advances in HIV-1 CTL epitope characterization. In HIV molecular immunology database. B.T.M. Korber et al., editors. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory. Los Alamos, New Mexico, USA. I1–I19.
- 29.Altfeld MA, et al. Identification of dominant optimal HLA-B60- and HLA-B61-restricted cytotoxic T-lymphocyte (CTL) epitopes: rapid characterization of CTL responses by enzyme-linked immunospot assay. J Virol. 2000;74:8541–8549. doi: 10.1128/jvi.74.18.8541-8549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altfeld M, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betts MR, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altfeld M, et al. VPR is preferentially targeted by cytotoxic T lymphocytes during HIV-1 infection. J Immunol. 2001;167:2743–2752. doi: 10.4049/jimmunol.167.5.2743. [DOI] [PubMed] [Google Scholar]
- 33.Pantaleo G, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 34.Pantaleo G, Graziosi C, Fauci AS. The role of lymphoid organs in the pathogenesis of HIV infection. Semin Immunol. 1993;5:157–163. doi: 10.1006/smim.1993.1019. [DOI] [PubMed] [Google Scholar]
- 35.Pantaleo G, et al. Accumulation of human immunodeficiency virus-specific cytotoxic T lymphocytes away from the predominant site of virus replication during primary infection. Eur J Immunol. 1997;27:3166–3173. doi: 10.1002/eji.1830271213. [DOI] [PubMed] [Google Scholar]
- 36.Betts MR, et al. Putative immunodominant human immunodeficiency virus-specific CD8(+) T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J Virol. 2000;74:9144–9151. doi: 10.1128/jvi.74.19.9144-9151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betts MR, et al. Human immunodeficiency virus type 1-specific cytotoxic T lymphocyte activity is inversely correlated with HIV type 1 viral load in HIV type 1-infected long-term survivors. AIDS Res Hum Retroviruses. 1999;15:1219–1228. doi: 10.1089/088922299310313. [DOI] [PubMed] [Google Scholar]
- 38.Ogg GS, et al. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalams SA, et al. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oxenius A, et al. Distribution of functional HIV-specific CD8 T lymphocytes between blood and secondary lymphoid organs after 8-18 months of antiretroviral therapy in acutely infected patients. AIDS. 2001;15:1653–1656. doi: 10.1097/00002030-200109070-00007. [DOI] [PubMed] [Google Scholar]
- 41.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 42.Zhang L, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 43.Mercado R, et al. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 44.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]