The MET protooncogene was discovered because of the ability of oncogenic Met to mediate chemically induced transformation of a human osteogenic sarcoma cell line (1). The normal product of this gene, Met, is an unusual receptor tyrosine kinase that can be distinguished from most other such proteins on the basis of its biosynthesis and its structural features. This transmembrane protein is synthesized as a single-chain precursor, which undergoes intracellular proteolytic cleavage at a basic amino acid site, yielding a disulfide-linked heterodimer. Its C-terminal, intracellular region contains a multifunctional docking site that binds to various signaling molecules. These features define a Met receptor tyrosine kinase family consisting of three related proteins, Met, Ron, and c-Sea, the last of which may be the chicken ortholog of Ron.
The ligand of the Met receptor is HGF, also known as scatter factor (2). HGF was discovered simultaneously as a mitogenic factor for liver cells and as a fibroblast-derived scattering/motility factor for epithelial cells. It is a multifunctional factor affecting a number of cell targets including epithelium, endothelium, myoblasts, spinal motor neurons, and hematopoietic cells. Signaling pathways activated by the HGF-Met interaction mediate cell adhesion and motility. As is argued by Trusolino and Comoglio (this Perspective series, ref. 3), these cellular phenotypes, coupled to tightly regulated changes in cell growth, morphology, and survival, define a general pattern of invasive growth that occurs widely in normal development.
In addition to regulating normal cell functions, Met is involved in malignant cell transformation. Increased Met expression has been found in papillary carcinomas of the thyroid gland, in carcinomas of colon, pancreas, and ovary, in osteogenic sarcomas, and in other types of cancer. Point mutations in MET have been identified in hereditary and sporadic papillary renal carcinomas (4–6), hepatocellular and gastric carcinomas (7, 8), and head and neck squamous carcinomas (9). Numerous experimental and clinical data indicate a particular role of HGF and Met in tumor invasive growth, a stage of tumor progression leading to metastases. Dysregulation of Met activity in cells is thought to be a key event underlying tumor metastasis, and indeed, Met overexpression and hyperactivation are reported to correlate with metastatic ability of the tumor cells (see sidebar, page 864).
Mechanisms leading to Met dysregulation in tumor cells
HGF binding to Met results in receptor autophosphorylation and upregulation of Met kinase activity, which in turn stimulates a number of intracellular pathways mediating HGF’s biological effects. In normal cells, Met activation is a ligand-dependent transient event, whereas in tumor cells Met activity is often constitutively upregulated.
Ligand-dependent mechanisms of Met activation.
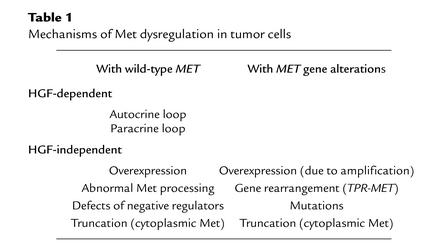
Met activation in tumor cells can occur through any of several molecular mechanisms (Table 1), the simplest of which involve HGF-dependent Met activation, much as occurs in normal cells. In some cases, tumor cells express both HGF and its receptor, setting the stage for an autocrine loop in which secreted HGF binds to Met and causes constitutive activation of Met and its downstream signaling pathways, thus enhancing tumor growth and invasive behavior. Such HGF-Met autocrine loops have been detected in gliomas, osteosarcomas, and mammary, prostate, breast, lung, and other carcinomas; they are often associated with malignant progression of tumors and correlate with poor prognosis. Interference with either HGF or Met expression can inhibit tumorigenic transformation, angiogenesis, tumor growth, and invasion (10).
Table 1.
Mechanisms of Met dysregulation in tumor cells
Under physiological conditions HGF is not an autocrine, but rather a paracrine, factor: Mesenchymal cells produce HGF, which acts on epithelial and other cells that express Met. Similarly, Met-positive tumor cells that do not produce HGF may nevertheless respond to HGF produced by stromal cells. However, since HGF is secreted by cells as a single-chain inactive precursor (pro-HGF), which must be activated by proteolytic cleavage, HGF-Met autocrine and paracrine loops depend on a third component — an enzyme capable of processing pro-HGF to produce HGF. A number of serine-like proteases, including urokinase-type plasminogen activator and coagulation factor XII, have such an activity and have been detected in some tumors. Nevertheless, the mechanism by which pro-HGF is converted to HGF in tumor tissues remains to be established.
Ligand-independent mechanisms.
Met can also be activated in an HGF-independent manner in tumors, particularly as a result of Met overexpression, which occurs in almost every case of differentiated papillary carcinomas. Increased Met expression can be mediated by MET gene amplification, by enhanced transcription, or by posttranscriptional mechanisms. Increased expression of Met on the cell surface apparently favors ligand-independent activation through spontaneous Met dimerization, but it is not generally sufficient to trigger Met activation. In some cases, even very high expression of Met does not cause constitutive receptor activation (11). Noncovalently associated, inactive clusters of these receptors have been identified on the cell surface, perhaps explaining the cells’ resistance to transformation, even in the face of high Met levels (12). An additional signal, such as Met transactivation by other membrane receptors, may be required to activate signaling by these receptors. Alternatively, these clusters may contain suppressor molecules that prevent spontaneous Met activation in normal cells but may be lost or inactivated in tumor cells.
One well-known oncogenic form of Met, first identified in the chemically transformed human osteosarcoma cell line HOS (1), is the product of the TPR-MET fusion, which arises through a chromosomal rearrangement. The resulting chimeric gene contains the promoter and the N-terminal sequence of the TPR gene from chromosome 1, fused with the C-terminal sequence of MET, which maps to chromosome 7. The TPR-MET chimeric gene encodes a cytoplasmic protein with molecular weight 65 kDa comprising the TPR leucine zipper domain and the Met kinase domain. This protein is constitutively active as a result of TPR leucine zipper interactions, which allow for Met kinase dimerization, transphosphorylation, and activation (13), and it is potently oncogenic in vitro and in vivo.
Abnormal processing or the absence of normal negative regulators can also lead to constitutive Met activation and tumorigenesis. The mature Met consists of two subunits, α and β, arising from proteolytic cleavage of the single-chain precursor. As a result of defective posttranslational processing, the precursor fails to be cleaved in the colon carcinoma cell line LoVo; consequently, Met is expressed on the cell surface as a single-chain molecule, which is constitutively tyrosine-phosphorylated (14). In metastatic B16 melanoma cells, on the other hand, cytosolic phosphatases that normally mediate Met dephosphorylation, internalization, and degradation are downregulated, leading to constitutive Met activation (15).
Finally, a large class of somatic or inherited mutations in the MET gene can lead to active, typically ligand-independent, Met signaling in tumor cells. For instance, a mutant in which the Met cytoplasmic domain is truncated immediately below the transmembrane domain encodes a constitutively active signaling domain that can transform rodent fibroblasts (16). A similar, naturally occurring truncation has been detected in malignant human musculoskeletal tumors. This short 85-kDa N-terminally truncated Met is tyrosine-phosphorylated and located in the cytoplasm (17). The mechanism by which this truncated Met is produced is not known, but its constitutive activation suggests a role in tumorigenesis.
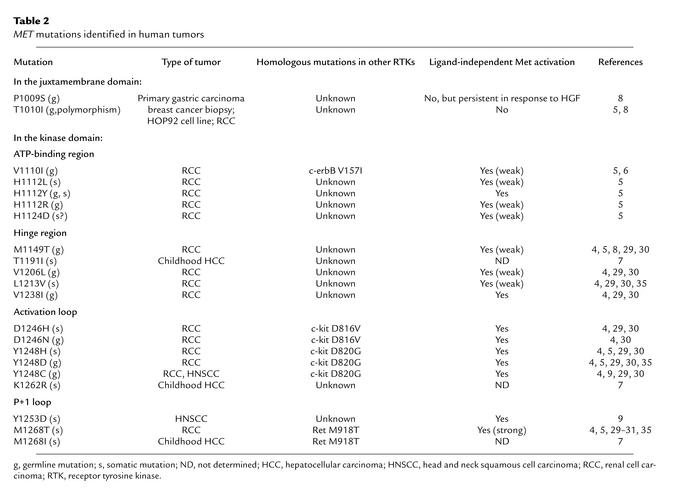
Missense point mutations in MET have been identified in hereditary and sporadic papillary renal carcinomas (4–6), childhood hepatocellular carcinomas (7), gastric carcinomas (9), and head and neck squamous cell carcinomas (8, 9). At present, 21 such mutations have been described, as summarized in Table 2. All identified Met mutations in the kinase domain increase Met tyrosine kinase activity. Although mutations in the juxtamembrane domain do not trigger ligand-independent Met activation, receptors carrying the P1009I mutation show persistent Met activation in response to HGF (7). This mutated form of Met demonstrates transforming potential and invasive activity in vitro and in vivo.
Table 2.
MET mutations identified in human tumors
Met transactivation via other membrane receptors.
Recent investigations have shown that Met kinase activity can be regulated through other receptors by HGF-independent mechanisms. Thus, Met can be activated by stimuli that do not directly interact with Met, including adhesive receptors, such as various integrins and CD44, and signal transducing receptors like Ron and the EGF receptor.
Integrins (discussed by Brakebusch et al., this Perspective series, ref. 18) are cell surface receptors that mediate cell adhesion to the ECM. Plating of Met-expressing cells on ECM, and the consequent ligation of cell surface integrins, can cause ligand-independent Met tyrosine phosphorylation (19). Interestingly, transgenic mice expressing Met in hepatocytes have activated Met and develop hepatocellular carcinoma (20), despite the absence of detectable HGF expression, perhaps as a result of cellular adhesion in this tissue (20).
CD44, a cell surface receptor for hyaluronic acid (a major glycosaminoglycan component of the ECM), regulates a number of normal cell functions and has been implicated in tumor progression and metastasis. This receptor can promote Met activation by two mechanisms. First, binding of CD44 to hyaluronic acid causes HGF-independent Met activation, leading to cell growth and migration (21). Second, a heparan sulfate proteoglycan isoform of CD44 binds HGF and presents it to Met in the form of a multivalent complex inducing a high level of Met activation in comparison with soluble nonbound HGF (22).
Because Ron belongs to the same family of receptor tyrosine kinases as Met and shares many common structural features, it is perhaps not surprising that activated Ron can transphosphorylate Met, and vice versa, as was recently shown (23). Pre-existing, ligand-independent heterodimers between Met and Ron have been detected on the cell surface (23), indicating that these receptors are colocalized and may be able to transphosphorylate and to activate one another. In addition, some human hepatoma cell lines — but not normal hepatocytes — are activated by a TGF-α–EGF receptor autocrine loop, which leads to constitutive, ligand-independent tyrosine phosphorylation of Met (24).
Consequences of Met dysregulation by various mechanisms
Although increased Met kinase activity represents a common feature of many tumors, the specific consequences of Met dysregulation are not uniform but reflect the molecular mechanism involved.
Cellular localization and the oncogenic potential of mutant Met.
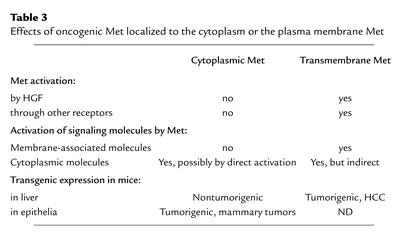
The effect of Met intracellular localization on its transforming ability has been investigated using the chimeric oncoprotein TPR-Met, which is found in soluble form in the cytoplasm but can be targeted to the cellular membrane by the addition of an Src myristoylation signal. Membrane-localized TPR-Met, but not cytoplasmic TPR-Met, stimulates the phosphatidylinositol 3-kinase–dependent (PI 3-kinase–dependent) induction of hyaluronic acid and its receptor and enhances cellular transformation (25).
Compared with two other, membrane-associated forms of oncogenic Met, the soluble TPR-Met chimera is much more dependent on its ability to interact with the binding site of the adaptor protein Grb2 for its biological activity (26). Transgenic expression of TPR-Met in the mouse liver causes hepatocyte immortalization and protects cells from apoptosis, but it does not result in tumor formation (27), whereas overexpression of the full-sized transmembrane form of Met induces hepatocellular carcinoma in transgenic mouse liver (20). Conversely, cytoplasmic TPR-Met is indeed oncogenic in mammary epithelial cells, since its transgenic expression induces mammary tumors in mice (28). Distinctions between transmembrane and cytoplasmic Met are described in Table 3.
Table 3.
Effects of oncogenic Met localized to the cytoplasm or the plasma membrane Met
The catalytic activity and substrate specificity of the Met M1268T mutant.
Point mutations in the kinase domain convert Met to an oncogenic receptor. Such mutants are highly active catalytically, which correlates with more efficient Met autophosphorylation, phosphorylation of substrates, and transforming ability (29, 30). For example, the constitutive binding of c-Src to the cytoplasmic domain of the Met M1268T mutant (identified in human renal papillary carcinomas) elevates c-Src phosphorylation and activity — an effect that is considerably more dramatic and longer-lasting than the transient activation of c-Src by HGF in normal cells. Dominant negative c-Src constructs effectively inhibit the oncogenic effect of Met M1268T, indicating that c-Src is required for transformation by this pathway (31). In NIH 3T3 cells, M1268 Met also activates the β-catenin pathway. Expression of Met M1268T mutant induces β-catenin tyrosine phosphorylation and accumulation; induces constitutive activation of the transcription factor Tcf, which acts in concert with β-catenin in the nucleus; and increases expression of the β-catenin/Tcf target genes Myc and Cyclin D1 (see Conacci-Sorrell et al., this Perspective series, ref. 32). Activation of the β-catenin pathway correlates with Met M1268T mutant–mediated cell transformation (33).
Although the hyperactive catalytic function of mutated Met is clearly associated with cellular transformation, a switch in substrate specificity may play a primary role in oncogenesis mediated by mutant forms of Met. In particular, Met M1268T phosphorylates substrates of the cytosolic kinase c-Abl, whereas wild-type Met does not (30), suggesting that mutated Met can activate signaling pathways distinct from those induced by wild-type Met. In addition to changes of substrate specificity, a set of phosphorylated tyrosine residues in wild-type and mutated Met molecules might be overlapping but distinct. These unique tyrosine phosphorylation sites may appear as a result of unusual conformations adopted by mutant Met kinase and may be phosphorylated by Met itself or by other kinases.
Catalytic and structural effects of Met missense mutations.
Analyses of three-dimensional structures of Met wild-type and mutated Met catalytic core domains show that mutations can activate Met by multiple mechanisms (34). Mutations such as V1110I, Y1248H/D/C, and M1268T directly affect contacts between residues in the protein’s activation loop; a region of the protein must undergo a regulated conformational change to permit the activation of the Met kinase. Mutations M1149T and L1213V may increase flexibility at the critical points of the Met tertiary structure leading to subdomain movements, whereas the D1246N mutation can stabilize the active form of the Met kinase (34). The M1268T mutation, which permits the efficient phosphorylation of c-Abl substrates (30), affects substrate binding sites within the Met kinase domain (30). These various structural changes are associated with functional diversity among oncogenic Met mutants. Thus, the D1246H/N and M1268T mutants have a high transforming ability, which correlates with activation of the Ras pathway, whereas the Y1248C and L1213V mutants are weakly transforming but promote cell migration, invasion, and resistance to apoptosis through activation of the PI 3-kinase/AKT pathway (35).
Some open questions
Data accumulating over the past few years represent significant progress in our understanding of the role of Met in oncogenesis, but a number of important questions remain. First, elucidating the mechanisms of Met dysregulation in various tumor types remains a high priority for both basic and clinical researchers. Met dysregulation may be a primary event in transformation, as a result of mutation, rearrangement, or amplification of the MET gene. In other cases, dysregulation of Met may be secondary to effects on other molecules. The study of signaling pathways by which Met expression and activity are regulated in normal cells and dysregulated in tumors suggests a number of promising therapeutic targets for anticancer drug development.
Another important direction for future investigations will involve solving the three-dimensional structure of Met, which will permit a clearer understanding of the structural and biological effects of the various Met kinase domain point mutations — particularly the pattern of tyrosine phosphorylation sites in Met and consequent switching of Met substrate specificity. Based on such analysis, it may be possible to develop agents that can selectively inhibit activity of the mutated Met kinase without interfering with the HGF-stimulated activity of normal Met.
Finally, it will be essential to deepen our understanding of receptor cross-talk and its contribution to Met activation and the propagation of Met-dependent oncogenesis. The dysregulated cell motility and the resulting tumor metastasis that follow from inappropriate Met activation involve collaborations with many other receptors and multiple signaling pathways. While the details remain obscure, it is clear that Met can function as part of other receptor complexes and can respond to stimuli that do not impinge on it directly. Conversely, dysregulated Met may promote activation of these other receptors. Both kinds of interactions undoubtedly have profound consequences for the invasive growth of tumors and, indeed, of healthy cells and tissues.
Supplementary Material
Acknowledgments
The authors would like to thank all researchers whose scientific contributions in the field helped to write this review. We apologize for failing to refer to many primary sources due to space limitations.
References
- 1.Cooper CS, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- 2.Bottaro DP, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 3.Comoglio PM, Trusolino L. Invasive growth: from development to metastasis. J Clin Invest. 2002;109:857–862. DOI:10.1172/JCI200215392. doi: 10.1172/JCI15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt L, et al. Germline and somatic mutations in the tyrosine kinase domain of the METproto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt L, et al. Novel mutations of the METproto-oncogene in papillary renal carcinomas. Oncogene. 1999;18:2343–2350. doi: 10.1038/sj.onc.1202547. [DOI] [PubMed] [Google Scholar]
- 6.Olivero M, et al. Novel mutation in the ATP-binding site of the METoncogene tyrosine kinase in a HPRCC family. Int J Cancer. 1999;82:640–643. doi: 10.1002/(sici)1097-0215(19990827)82:5<640::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Park WS, et al. Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res. 1999;59:307–310. [PubMed] [Google Scholar]
- 8.Lee JH, et al. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene. 2000;19:4947–4953. doi: 10.1038/sj.onc.1203874. [DOI] [PubMed] [Google Scholar]
- 9.Di Renzo MF, et al. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene. 2000;19:1547–1555. doi: 10.1038/sj.onc.1203455. [DOI] [PubMed] [Google Scholar]
- 10.Abounader R, et al. Reversion of human glioblastoma malignancy by U1 small nuclear RNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c-met expression. J Natl Cancer Inst. 1999;91:1548–1556. doi: 10.1093/jnci/91.18.1548. [DOI] [PubMed] [Google Scholar]
- 11.Ferracini R, et al. The Met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene. 1995;10:739–749. [PubMed] [Google Scholar]
- 12.Faletto DL, et al. Evidence for non-covalent clusters of the c-met proto-oncogene product. Oncogene. 1992;7:1149–1157. [PubMed] [Google Scholar]
- 13.Rodrigues GA, Park M. Dimerization mediated through a leucine zipper activates the oncogenic potential of the met receptor tyrosine kinase. Mol Cell Biol. 1993;13:6711–6722. doi: 10.1128/mcb.13.11.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondino A, Giordano S, Comoglio PM. Defective posttranslational processing activates the tyrosine kinase encoded by the MET proto-oncogene (hepatocyte growth factor receptor) Mol Cell Biol. 1991;11:6084–6092. doi: 10.1128/mcb.11.12.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rusciano D, Lorenzoni P, Burger MM. Constitutive activation of c-Met in liver metastatic B16 melanoma cells depends on both substrate adhesion and cell5 density and is regulated by a cytosolic tyrosine phosphatase activity. J Biol Chem. 1996;271:20763–20769. doi: 10.1074/jbc.271.34.20763. [DOI] [PubMed] [Google Scholar]
- 16.Zhen Z, et al. Structural and functional domains critical for constitutive activation of the HGF-receptor (Met) Oncogene. 1994;9:1691–1697. [PubMed] [Google Scholar]
- 17.Wallenius V, et al. Overexpression of the hepatocyte growth factor (HGF) receptor (Met) and presence of a truncated and activated intracellular HGF receptor fragment in locally aggressive/malignant human musculoskeletal tumors. Am J Pathol. 2000;156:821–829. doi: 10.1016/S0002-9440(10)64950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brakebusch, C., Bouvard, D., Stanchi, F., Sakai, T., and Fässler, R. 2002. Integrins in invasive growth. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 19.Wang R, Kobayashi R, Bishop JM. Cellular adherence elicits ligand-independent activation of the Met cell-surface receptor. Proc Natl Acad Sci USA. 1996;93:8425–8430. doi: 10.1073/pnas.93.16.8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–1034. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taher TE, et al. Cross-talk between CD44 and c-Met in B cells. Curr Top Microbiol Immunol. 1999;246:31–37. doi: 10.1007/978-3-642-60162-0_4. [DOI] [PubMed] [Google Scholar]
- 22.Van der Voort R, et al. Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met. J Biol Chem. 1999;274:6499–6506. doi: 10.1074/jbc.274.10.6499. [DOI] [PubMed] [Google Scholar]
- 23.Follenzi A, et al. Cross-talk between the proto-oncogenes Met and Ron. Oncogene. 2000;19:3041–3049. doi: 10.1038/sj.onc.1203620. [DOI] [PubMed] [Google Scholar]
- 24.Jo M, et al. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 25.Kamikura DM, Khoury H, Maroun C, Naujokas MA, Park M. Enhanced transformation by a plasma membrane-associated met oncoprotein: activation of a phosphoinositide 3′-kinase-dependent autocrine loop involving hyaluronic acid and CD44. Mol Cell Biol. 2000;20:3482–3496. doi: 10.1128/mcb.20.10.3482-3496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffers M, Koochekpour S, Fiscella M, Sathyanarayana BK, Vande WG. Signaling requirements for oncogenic forms of the Met tyrosine kinase receptor. Oncogene. 1998;17:2691–2700. doi: 10.1038/sj.onc.1202209. [DOI] [PubMed] [Google Scholar]
- 27.Amicone L, et al. Transgenic expression in the liver of truncated Met blocks apoptosis and permits immortalization of hepatocytes. EMBO J. 1997;16:495–503. doi: 10.1093/emboj/16.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang TJ, Reid AE, Xavier R, Cardiff RD, Wang TC. Transgenic expression of tpr-met oncogene leads to development of mammary hyperplasia and tumors. J Clin Invest. 1996;97:2872–2877. doi: 10.1172/JCI118744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeffers M, et al. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci USA. 1997;94:11445–11450. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardelli A, et al. Uncoupling signal transducers from oncogenic MET mutants abrogates cell transformation and inhibits invasive growth. Proc Natl Acad Sci USA. 1998;95:14379–14383. doi: 10.1073/pnas.95.24.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakaigawa N, Weirich G, Schmidt L, Zbar B. Tumorigenesis mediated by MET mutant M1268T is inhibited by dominant-negative Src. Oncogene. 2000;19:2996–3002. doi: 10.1038/sj.onc.1203628. [DOI] [PubMed] [Google Scholar]
- 32.Conacci-Sorrell, M., Zhurinsky, J., Ben-Ze’ev, A. 2002. The cadherin-catenin adhesion system in signaling and cancer. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 33.Danilkovitch-Miagkova A, et al. Oncogenic mutants of RON and MET receptor tyrosine kinases cause activation of the β-catenin pathway. Mol Cell Biol. 2001;21:5857–5868. doi: 10.1128/MCB.21.17.5857-5868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller M, et al. Structural basis of oncogenic activation caused by point mutations in the kinase domain of the MET proto-oncogene: modeling studies. Proteins. 2001;44:32–43. doi: 10.1002/prot.1069. [DOI] [PubMed] [Google Scholar]
- 35.Giordano S, et al. Different point mutations in the met oncogene elicit distinct biological properties. FASEB J. 2000;14:401–408. doi: 10.1096/fasebj.14.2.399. Table Mechanisms of Met dysregulation in tumor cellsWith wild-type METWith MET gene alterationsHGF-dependentAutocrine loopParacrine loopHGF-independentOverexpressionOverexpression (due to amplification)Abnormal Met processingGene rearrangement (TPR-MET)Defects of negative regulatorsMutationsTruncation (cytoplasmic Met)Truncation (cytoplasmic Met) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.