Abstract
Susceptibility to myasthenia gravis (MG) is positively linked to expression of HLA-DQ8 and DR3 molecules and negatively linked to expression of the DQ6 molecule. To elucidate the molecular basis of this association, we have induced experimental autoimmune MG (EAMG) in mice transgenic for HLA-DQ8, DQ6, and DR3, and in DQ8×DQ6 and DQ8×DR3 F1 transgenic mice, by immunization with human acetylcholine receptor (H-AChR) in CFA. Mice expressing transgenes for one or both of the HLA class II molecules positively associated with MG (DQ8 and DR3) developed EAMG. T cells from DQ8 transgenic mice responded well to three cytoplasmic peptide sequences of H-AChR (α320-337, α304-322, and α419-437), of which the response to α320-337 was the most intense. DR3 transgenic mice also responded to this sequence very strongly. H-AChR– and α320-337 peptide–specific lymphocyte responses were restricted by HLA class II molecules. Disease resistance in DQ6 transgenic mice was associated with reduced synthesis of anti-AChR IgG, IgG2b, and IgG2c Ab’s and reduced IL-2 and IFN-γ secretion by H-AChR– and peptide α320-337–specific lymphocytes. Finally, we show that DQ8 imparts susceptibility to EAMG and responsiveness to an epitope within the sequence α320-337 as a dominant trait.
Introduction
The human autoimmune disease myasthenia gravis (MG) is characterized by T cell and Ab responses to muscle nicotinic acetylcholine receptor (AChR). High-affinity, anti-AChR Ab’s, upon binding at the neuromuscular junction, activate complement and accelerate AChR destruction, while causing failure of neuromuscular transmission and the resulting myasthenic symptoms (1–3). However, AChR-specific CD4+ T cells, which can be detected in most MG patients (3), likely have an important role in MG, because they modulate the synthesis of anti-AChR Ab and may be the prime movers in the pathogenesis of MG. Experimental autoimmune MG (EAMG) is a model of MG, and it is induced in experimental animals by immunization with purified AChR from different sources. Immunization of C57BL/6 mice with Torpedo californica AChR (T-AChR) in CFA causes activation of specific T and B cells, and synthesis of anti-AChR Ab (4). As in MG, the anti-AChR Ab’s bind at the mouse neuromuscular junction, activate the complement cascade, and cause increased AChR loss and EAMG (1, 3, 5, 6). MHC class II gene products influence the T cell and Ab response to the AChR, and susceptibility to EAMG (7, 8). In the B6.C-H-2bm12 (bm12) strain, a gene conversion event between I-Eβb and I-Aβb altered three amino acids in the C-terminal half of the first domain of Aβb (Ile67→Phe; Arg70→Gln; Thr71→Lys) and resulted in relative resistance to EAMG development (9). In C57BL/6 mice, depletion of CD4+ cells in vivo prevented EAMG and also induced clinical remission, demonstrating the critical role of CD4+ cells in EAMG pathogenesis (10).
HLA-DQβ chain polymorphism and increased frequency of HLA-DQ8 and HLA-DR3 alleles have been associated with human MG susceptibility (11, 12). On the other hand, the DQ6 allele has been suggested to be negatively associated with MG (13). To study the role of human HLA class II molecules in MG, Raju et al. examined the susceptibility to EAMG in B10 mice transgenic for human HLA-DQ8 and DQ6 class II molecules (14). The HLA transgenic mice do not have the endogenous I-Aβ and I-Eα molecules and therefore only express human HLA-DQ8, DQ6, or DR3 transgenic class II molecules (14, 15). HLA transgenic mice developed EAMG following immunization with T-AChR (14, 15). Further, transgenic expression of HLA-DQ6 correlated with reduced susceptibility to EAMG (14).
In the EAMG-susceptible C57BL/6 (I-Ab) mice immunized with T-AChR, the sequence region 146–169 of the T-AChR α subunit forms an immunodominant epitope for CD4+ T cell sensitization (16–19). Several studies have suggested that sensitization of CD4+ cells to epitopes within that immunodominant sequence region was important for EAMG development. The AChR-immune lymphocytes of bm12 mice exhibited a reduced proliferative response to epitopes within the α146-169 sequence (16–19). Neonatal tolerance to T-α146-162 peptide, and nasal or systemic administration to adult C57BL/6 mice of synthetic peptides within the sequence T-α146-169, prevented EAMG development after T-AChR immunization (19–22). When using systemic high doses of a T-α146-162 peptide, the resulting tolerance was mediated by the Fas/Fas ligand pathway, apoptosis, and clonal anergy of AChR-reactive CD4+ cells (23). Unlike T cells from wild-type C57BL/6 mice, those from HLA-DQ8 and HLA-DR3 transgenic mice immunized with T-AChR recognized, primarily, two promiscuous determinants within residues 141–160 and 170–190 of the human AChR (H-AChR) α subunit (15).
In this study, we induced EAMG by H-AChR immunization and characterized the H-AChR T cell epitopes involved in activation of T cells, and the cytokines secreted by the anti–H-AChR T cells. Toward that goal, we immunized HLA transgenic mice with H-AChR derived from the human muscle–like TE671 cell line. H-AChR–immunized HLA-DQ8, DR3, DQ8×DR3 F1, and DQ8×DQ6 F1 mice developed clinical EAMG, whereas HLA-DQ6 mice were less susceptible. The cytoplasmic H-AChR peptide α320-337 (H-α320-337) was the dominant T cell epitope for DQ8, DR3, and DQ8×DQ6 F1 mice. The HLA class II–restricted lymphocyte response against H-AChR and peptide H-α320-337 and susceptibility to EAMG were inherited as a dominant trait in DQ8×DQ6 F1 mice.
Methods
Mice.
We used transgenic mice deficient in the mouse MHC class II molecules, which bear only a human HLA-DQ (Aβ0.DQ8 or Aβ0.DQ6) or DR (Aβ0.DR3) molecule. The mice express functional HLA-DQ8 (HLA-DQA1*0301/DQB1*0302), DQ6 (HLA-DQA1*0103/DQB1*0601), or DR3 (HLA-DRA1*0101/DRB1*0301) genes in the C57BL/10 background. All of the transgenic mice, except DQ8×DQ6 F1 mice, were obtained after the tenth backcross generation to C57BL/10 mice. The Aβ0.DR3 (DR3) mice were mated with Aβ0.DQ8 (DQ8) mice to obtain the DQ8×DR3 F1 mice. The fourth backcross generation of Aβ0.DQ8 and Aβ0.DQ6 (DQ6) mice were mated to derive DQ8×DQ6 F1 mice. In all of the transgenic mice, expression of HLA class II and absence of endogenous MHC class II molecules were verified by FACS and PCR (14, 15, 24, 25). The percentage of CD4+ and CD8+ cells in the peripheral blood leukocytes of transgenic mice before immunization was analyzed by flow cytometry as previously demonstrated (25). CD4+ cells in the different strains were: DQ6, 15.68% ± 3.55%; DQ8, 14.02% ± 2.20%; DR3, 15.73% ± 3.03%; and DQ8×DR3 F1, 16.08% ± 4.41%; while CD8+ cells were as follows: DQ6, 10.14% ± 1.12%; DQ8, 11.42% ± 2.76%; DR3, 11.34% ± 4.89%; and DQ8×DR3 F1, 10.6% ± 1.60%. No significant differences between these strains of mice were found. Therefore, the immune system in HLA transgenic mice developed normally, with appropriate expression and functionality of MHC class II molecules. All transgenic lines were initially bred at the Mayo Clinic and then bred and housed in the viral Ab–free barrier facility at the University of Texas Medical Branch. All experiments were performed according to the Animal Care and Use Committee guidelines.
Purification of human AChR, biological activity, and SDS-PAGE.
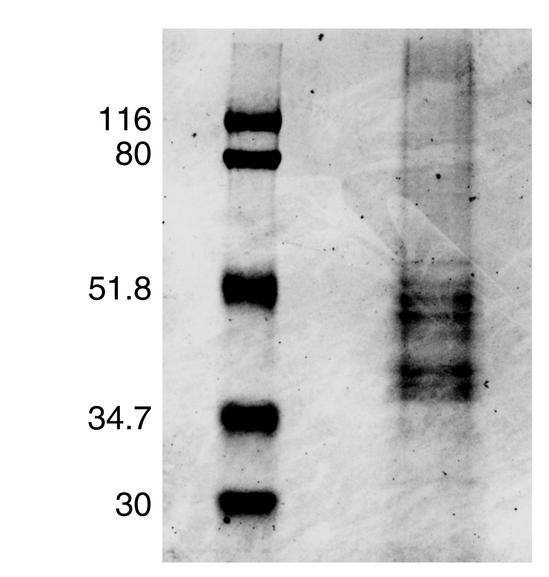
H-AChR was purified from the TE671 cell line (American Type Culture Collection, Rockville, Maryland, USA), which expresses H-AChR, by an α-neurotoxin affinity column. The TE671 cell line was cultured in 150-cm2 cell culture flasks at 37°C in Dulbecco’s modification of Eagle’s medium supplemented with 2 mM/l L-glutamine, and 10% of FBS in the presence of 100 U/ml penicillin G and 100 μg/ml streptomycin. Confluent cells were harvested every 3–4 days. Cells were washed in homogenization buffer containing 5 mM/l Tris (pH 7.8), 10 mM/l EDTA, 10 mM/l EGTA, 0.1 g/l phenylmethylsulfonyl fluoride, and 0.2 g/l sodium azide. The cells were removed from the flask, and cells collected from 70–100 flasks were used for one affinity purification procedure. After washing, the pellet was resuspended in a small amount of homogenizing buffer, and 0.1 vol of 10% Triton (1% Triton X-100) was added to the TE671 cells and agitated for 4 hours on a shaker at low speed at 4°C. The extract was centrifuged for 30 minutes at 100,000 g at 4°C. AChR containing supernatant was added to neurotoxin 3–agarose (Sigma Chemical Co., St. Louis, Missouri, USA) in a glass flask and agitated gently in a shaker for 4 hours at 4°C. The next day, AChR extract/neurotoxin 3–agarose mixture was poured into a 1.5 × 20-cm Econo column (Bio-Rad Laboratories, Life Science Group, Hercules, California, USA). The mixture was poured down the sides of the column and the buffer allowed to run out of the column. After beads were packed, supernatants were allowed to run out of the column until a small amount covered the top. NaCl/Triton buffer was added to the top, and the column was washed with 100 ml of buffer. Following this step, both the toxin affinity and hydroxylapatite columns were washed with 300 ml 0.2% cholate buffer. Buffer from the top of both columns was removed, and 1 M carbamylcholine buffer (26) (Sigma Chemical Co.) was added. Then the hydroxylapatite column, but not the neurotoxin 3 column, was washed with 100 ml of 1 M carbamylcholine buffer. Plastic tubing from both the columns was connected to a pump. The peristaltic pump was run at a rate of 50 ml/h, and the mixture was allowed to circulate for 14–16 hours. The connection between the toxin affinity and the hydroxylapatite columns was removed, and H-AChR was eluted with 152 mM phosphate elution buffer (26) into a fraction collector. The total yield from each purification varied with an average of 5 mg pure H-AChR protein from 40 to 60 g TE671 cells. We evaluated the ability of the H-AChR preparations to bind specifically to [125I]α-bungarotoxin ([125I]α-BTX). One microgram of affinity-purified H-AChR had 92–219 nM of α-BTX–binding sites. SDS-PAGE confirmed the purity of fetal H-AChR preparations with appropriate molecular weight of α (in duplicate), β, γ, and δ subunits (Figure 1) (27).
Figure 1.
SDS-PAGE separation of affinity-purified H-AChR protein. Standard protein markers and 20 μl of sample buffer containing 10 μg H-AChR protein were loaded on a 7 × 8-cm gel. PAGE was run in Mini-Protean II Electrophoresis Cell (Bio-Rad Laboratories) at 50 milli-Ampere for 2 hours and stained with Coomassie blue.
Peptides.
The peptides used in this study were synthesized by parallel synthesis (28). Most of them were 14–20 residues long and overlapped by three to five residues. They spanned most of the H-AChR α subunit sequence. Their position in the AChR α subunit and their sequences are reported in Table 1. The synthesis and characterization of these peptides have been reported (29–33). They were successfully used to identify the epitope repertoire of CD4+ cells from MG patients (29–33).
Table 1.
Human AChR α chain overlapping synthetic peptidesA
Induction of EAMG and clinical evaluation of the disease.
DQ8, DR3, DQ6 (eight mice each), and ten DQ8×DR3 F1 mice were immunized with H-AChR (20 μg per mouse) in CFA on days 0, 30, and 60. The mice were evaluated for clinical EAMG, and their muscle weakness was graded as follows. Grade 0: normal strength without any muscle weakness; grade 1: definite weakness of the forelimbs with a characteristic hunched, chin-down posture and flaccid tail, after exercise involving 20–30 consecutive paw grips on the top steel grids of the animal cage; grade 2: grade 1 weakness at rest; grade 3: severe weakness with paralysis involving all the limbs (quadriplegia) accompanied by dehydration and moribundity. Serum from individual mice was collected before first immunization and on days 15, 45, 75, and 90 after first immunization.
Lymphocyte proliferative response in vitro to the H-AChR and mapping of H-AChR epitopes recognized by T cells in DQ8, DQ6, DR3, and DQ8×DQ6 F1 mice.
DQ8, DQ6, DR3, and DQ8×DQ6 F1 transgenic mice were immunized in the footpads with 20 μg H-AChR in CFA. After 7 days, pooled draining (popliteal and inguinal) lymph node cells (LNCs) at 4 × 105 cells in 200 μl were exposed in triplicate to each of the H-AChR-α (H-α) subunit peptides (40 μg/ml) and H-AChR (0.5 μg/ml). Inguinal, paraortic, and axillary LNCs of individual mice derived on day 90 of the long-term experiment were cultured in triplicate with H-α320-337 peptide (40 μg/ml) or H-AChR (0.5 μg/ml). The culture medium contained RPMI-1640, supplemented with 10% FCS, penicillin G (100 U/ml) streptomycin (100 μg/ml), L-glutamine (2 mM), 2-mercaptoethanol (3 × 10–5 M), and HEPES buffer (25 mM). The cells were cultured for 5 days at 37°C in humidified 5% CO2-enriched air, and pulse-labeled with [3H]TdR (1 μCi per well) for 18–20 hours before harvesting. The 3H incorporation was determined in a Beckman beta scintillation counter (Beckman Coulter Inc., Fullerton, California, USA). The results are expressed as Δcpm (cpm with antigen minus cpm with media).
Anti-DQ and -DR Ab inhibition studies.
DR3 and DQ8 mice (four of each) were immunized with H-AChR (20 μg per mice) in CFA. A week later, the mice were euthanized, and the pooled draining popliteal and inguinal LNCs in triplicate wells were cultured for 5 days with H-AChR (0.5 μg/ml), with or without mAb’s specific for HLA-DQ (Leinco Technologies Inc., St. Louis, Missouri, USA; clone 1a3) or HLA-DR molecules (PharMingen, San Diego, California, USA; clone G46-6, dialyzed to remove sodium azide). The extent of cell proliferation was determined from the incorporation of 3H-thymidine.
Serum anti-AChR Ab assay.
To detect cross-reactive auto-Ab to mouse muscle AChR (M-AChR), crude mouse muscle extract containing M-AChR was incubated in Triton buffer with [125I]α-BTX (5 × 10–9 M; Amersham Life Sciences Inc., Arlington Heights, Illinois, USA) for 1 hour. One microliter of serum of each mouse was added. Normal mouse serum (bled from mice before first immunization) served as control. After overnight incubation at 4°C, goat anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA) was added to 1 ml of [125I]α-BTX–labeled AChR. After 4 hours, the tubes were centrifuged, and the pellets were washed with 1 ml of Triton buffer, centrifuged again, and counted in a gamma counter. The AChR precipitated, minus the control value, gave the serum Ab amounts in nanomoles (nM) of α-BTX–binding sites bound per liter of serum.
ELISA of anti–M-AChR Ig isotype.
Affinity-purified M-AChR (0.5 μg/ml) was coated onto a 96-well microtiter plate in 0.1 M carbonate bicarbonate buffer (pH 9.6) overnight at 4°C. The plates were blocked with 2% BSA in PBS at room temperature for 30 minutes. Diluted serum samples of 100 μl (IgG, IgG1, IgG2b, and IgG2c 1:2500; IgM 1:100) were added and incubated at 37°C for 90 minutes. After four washes, horseradish peroxidase–conjugated anti-mouse IgM, IgG, IgG1, and IgG2b (Caltag Laboratories, Burlingame, California, USA) and anti-mouse IgG2c (PharMingen), diluted 1:1000 in PBS 0.05% Tween-20, were added and incubated at 37°C for 90 minutes. Subsequently, 0.3 mg/ml of diammonium 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonate) substrate (ABTS) solution was added to the IgM, IgG, IgG1, and IgG2b plates and 100 μl of avidin-labeled peroxidase (Sigma Chemical Co.) at 2.5 μg/ml was added to the IgG2c plate and incubated for 30 minutes. Subsequently, the peroxidase indicator substrate ABTS, in 0.1 M citric buffer, pH 4.35, was added in the presence of H2O2, and the mixture was allowed to develop color at room temperature in the dark. Plates were read at a wavelength of 405 nm. Serially diluted anti-AChR sera were used as a positive control, and normal mouse serum (collected from mice before H-AChR immunization) was used for background determination. The results are expressed as ΔOD (OD from sera after AChR immunization minus OD from sera before AChR immunization).
Radioimmunoassay of M-AChR content.
0.1-ml aliquots of [125I]α-BTX–labeled (5 × 10–9 M), Triton X-100–solubilized mouse muscle extract, both with and without benzoquinonium (10–3 M), were mixed with 10 μl of anti-AChR serum. The resulting complex was precipitated by goat anti-mouse serum and then sedimented by centrifugation, washed in 1 ml of Triton buffer, and sedimented again. Radioactivity of the pellet was counted in a Packard gamma counter (Packard Instrument Co., Meriden, Connecticut, USA), and cpm with benzoquinonium was subtracted from cpm of experimental samples without benzoquinonium. The concentration of AChR was expressed as pM of [125I]α-BTX–binding sites per gram of mouse carcass.
ELISA of cytokines secreted by cultured LNCs.
At the end of the long-term experiment, draining LNCs (inguinal, paraaortic, and axillary) from individual mice were cultured in 48-well, flat-bottomed plates in the presence of H-AChR (0.5 μg /ml) or H-α320-337 peptide (40 μg/ml); and at 48, 72, or 96 hours, the supernatant was collected for IL-2, IL-10, and IFN-γ measurement. Wells in ELISA plates (Immunol 2; Dynatech Laboratories, Chantilly, Virginia, USA) were coated with 2 μg/ml (50 μl per well) of one of the anti-cytokine (IL-2, IFN-γ, and IL-10; PharMingen) mAb’s in 0.1 M carbonate buffer, pH 8.2 overnight at 4°C. The plates were blocked with 200 μl of 10% FCS in PBS for 2 hours. Supernatant sample (100 μl) and various dilutions of cytokine standards (PharMingen) were added, titered to the linear portion of the absorbance/concentration curve in duplicate, and incubated overnight at 4°C. After the plates were washed six times with PBS and Tween-20 (0.05%), 100 μl biotinylated anti-cytokine mAb (with different epitopic determinants than the Ab used to coat ELISA plates) at 1 μg/ml in PBS containing 10% FCS were added for 45 minutes at room temperature. After vigorous washing, 100 μl of avidin-labeled peroxidase (Sigma Chemical Co.) at 2.5 μg/ml was added and incubated for 30 minutes. Subsequently, the peroxidase indicator substrate ABTS in 0.1 M citric buffer, pH 4.35, was added in the presence of H2O2, and the OD was measured at 405 nm using the ELISA reader from Molecular Devices Corp. (Sunnyvale, California, USA). The negative (background) controls were culture medium. The concentration of specific cytokine samples was measured based on cytokine standards (IFN-γ and IL-10, PharMingen; IL-2, R&D Systems Inc., Minneapolis, Minnesota, USA).
Results
H-AChR immunization induced clinical EAMG in DQ8, DR3, DQ8×DR3 F1, and DQ8×DQ6 F1 mice.
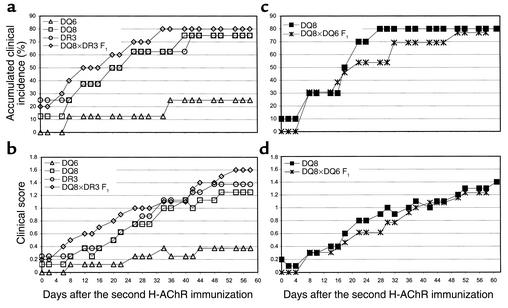
DQ6 mice were relatively resistant to clinical EAMG when immunized with T-AChR (14). In our study, before the second AChR immunization, two of eight DR3 mice (25%) and one of eight DQ8 mice (12.5%) developed grade 1 EAMG. After the second immunization, five of eight DQ8 (62.5%) and four of eight DR3 mice (50%) developed grade 1 or 2 EAMG. The accumulated total clinical incidence at the end of the experiment was six of eight (75%) for DQ8 and DR3 mice. The time course of the accumulated incidence of clinical EAMG and the time of onset of the clinical disease are illustrated in Figure 2a and the time course of the clinical score (mean grade) is illustrated in Figure 2b. Only two of eight DQ6 mice (25%) developed low-grade, clinical EAMG after the third immunization. Therefore, DQ6 mice are relatively resistant to EAMG and develop less severe disease when immunized with either H-AChR (this study) or T-AChR (14).
Figure 2.
(a and b) Kinetics of the accumulated clinical EAMG incidence (a) and mean clinical score (b) in DQ6, DQ8, DR3, and DQ8×DR3 F1 transgenic mice. DQ6 mice’s mean clinical scores (severity) were significantly lower than those of DQ8 and DR3 (from day 42 to the end of the experiment) and DQ8×DR3 F1 mice (from day 20 to the end of the experiment) after a second H-AChR immunization. P < 0.05, Student’s t test. In Fisher’s exact probability test, the difference in the clinical incidence between DQ8 and DQ6, and DR3 and DQ6 was significant at P = 0.060. (c and d) Clinical EAMG incidence (c) and mean clinical score (d) of DQ8 and DQ8×DQ6 F1 transgenic mice.
Since both DQ8 and DR3 alleles are positively associated with MG, we examined the susceptibility to EAMG of DQ8×DR3 F1 mice, which express both the DQ8 and the DQ3 molecules. Eight of ten DQ8×DR3 F1 mice (80%) developed clinical EAMG, and one died at 44 days after the second immunization because of severe disease (Figure 2, a and b). Expression of I-EαKβb (I-E) molecule (equivalent to human DR molecule) in B10 mice suppressed EAMG development (34). However, in HLA transgenic mice, expression of the DR3 molecule in the presence of EAMG susceptible DQ8 molecule did not suppress clinical EAMG.
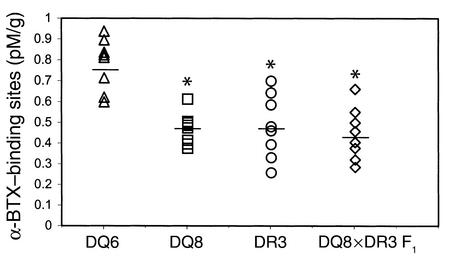
Next we examined whether simultaneous inheritance of the EAMG-susceptible DQ8 allele and the EAMG-resistant DQ6 allele leads to dominant susceptibility to EAMG. DQ8×DQ6 F1 mice developed clinical EAMG with an incidence and severity similar to those of DQ8 mice (Figure 2, c and d), suggesting the dominance of the DQ8 gene in determining EAMG susceptibility. Consistent with the clinical findings, the AChR content of mouse muscle, determined as α-BTX–binding sites, was significantly lower in H-AChR–immunized DQ8, DR3, and DQ8×DR3 F1 mice than in the resistant DQ6 mice (Figure 3).
Figure 3.
Muscle AChR content in AChR-immunized transgenic mice. Individual mouse carcasses were extracted to measure muscle AChR (α-BTX–binding sites) by radioimmunoassay and expressed as pM α-BTX–binding sites per gram of mouse carcass. DQ6 mice had increased amounts of functional muscle AChR compared with DQ8, DR3, and DQ8×DR3 F1 mice. *P < 0.01, Student’s t test. There was no difference in the muscle AChR content between normal DQ8 (1.16 ± 0.10 pM/g), DQ6 (0.94 ± 0.07 pM/g), and DR3 (1.25 ± 0.08 pM/g) mice.
Reduced serum anti-AChR Ab level in DQ6 mice, compared with DQ8, DR3, and DQ8×DR3 F1 mice.
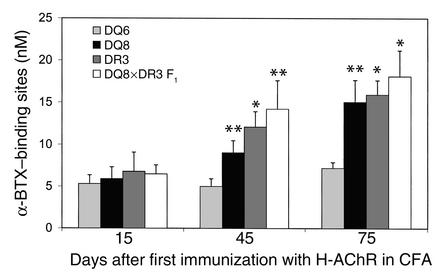
Serum collected on days 15, 45, and 75, after the beginning of the H-AChR immunization, were evaluated for anti–M-AChR Ab concentration by an α-BTX radioimmunoassay. The anti-AChR Ab response of all the transgenic strains was low on day 15 after priming with H-AChR, and no differences were observed between transgenic mice (Figure 4). However, at 45 days (15 days after the first H-AChR boost) and at 75 days (15 days after second boost), DQ8, DR3, and DQ8×DR3 F1 mice had significantly higher concentrations of serum anti–M-AChR Ab than did DQ6 mice (Figure 4). Therefore, the resistance of DQ6 mice to development of clinical EAMG could be due to the reduced production of cross-reactive Ab to mouse muscle AChR.
Figure 4.
Reduced anti-AChR IgG response in DQ6 mice compared with DQ8, DR3, and DQ8×DR3 F1 mice. Serum individually derived from DQ6, DQ8, DR3, and DQ8×DR3 F1 mice (eight mice of each group) on days 15, 45, and 75 after the first H-AChR immunization was measured for anti–mouse muscle AChR IgG level by α-BTX radioimmunoassay. The mean value of cpm of prebleeding serum from all transgenic mice was similar (DQ8, 396 ± 55; DR3, 414 ± 47; DQ6, 390 ± 30; DQ8×DR3 F1, 400 ± 47). The error bars are SE. *P < 0.01 and **P < 0.05, Student’s t test.
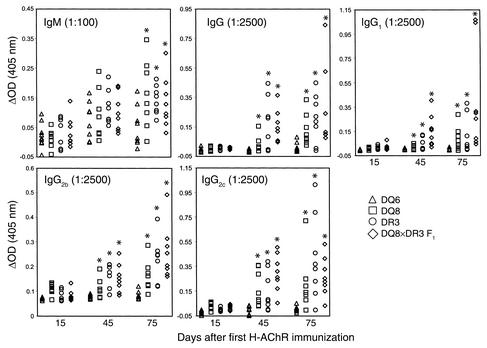
Reduced serum concentrations of anti-AChR IgG, IgG1, IgG2b, and IgG2c in DQ6 mice compared with DQ8, DR3, and DQ8×DR3 F1 mice.
Next we examined whether the concentration in the serum of anti-AChR IgG isotypes correlated with development of EAMG in the EAMG-susceptible DQ8, DR3, and DQ8×DR3 F1 mice, and in the relatively resistant DQ6 mice. Fifteen days after priming with H-AChR, we did not observe significant differences in the primary anti-AChR IgM Ab between DQ6 mice, and DQ8, DR3, and DQ8×DR3 F1 mice (Figure 5). However, the concentration of serum anti-AChR IgM Ab level was very low in all of the transgenic mice at that time. On day 75 (after two booster doses of H-AChR), DQ8, DR3, and DQ8×DR3 F1 mice had significantly higher serum anti-AChR IgM responses than did DQ6 mice (Figure 5). As observed for the α-BTX radioimmunoassay of anti-AChR Ab, also in the ELISA, the serum anti-AChR IgG Ab response was reduced in the DQ6 mice as compared with the DQ8, DR3, and DQ8×DR3 F1 mice (Figure 5). On days 45 and 75 (after one and two boosts with H-AChR, respectively), DQ6 mice had significantly reduced serum concentrations of anti-AChR IgG1, IgG2b, and IgG2c isotypes. Therefore, the relative resistance of EAMG in DQ6 mice was associated with reduced secondary response involving anti-AChR IgG1, IgG2b, and IgG2c Ab isotypes.
Figure 5.
Suppressed secondary anti-AChR IgG, IgG1, IgG2b, and IgG2c Ab in DQ6 mice. Anti-mouse AChR isotype in serum was measured by ELISA on affinity-purified, mouse AChR–coated plates. ΔOD (405 nM), the value from immunized mouse sample, was subtracted from the preimmunized value. *P > 0.05, Student’s t test.
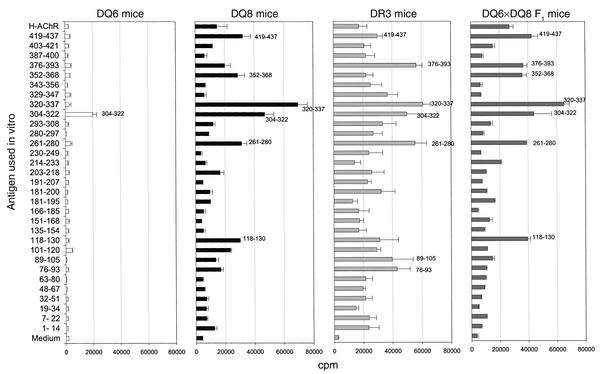
The sequence α320-337 of the H-AChR forms a dominant T cell epitope for DQ8, DR3, and DQ8×DQ6 F1 mice.
We next mapped the T cell epitopes on the H-AChR α subunit by challenging LNCs from H-AChR–immunized DQ8, DR3, DQ6, and DQ8×DQ6 F1 mice, with overlapping synthetic peptides spanning most of the sequence of the H-AChR α subunit. Peptide α320-337 was most strongly recognized by T cells of H-AChR–immunized DQ8, DR3, and DQ8×DQ6 F1 mice (Figure 6). Peptide α304-322 was a subdominant epitope in DQ8 mice. H-AChR–immunized T cells of DR3 mice responded well (>40,000 cpm) to numerous peptides of the H-AChR α subunit (α320-337, α376-393, α304-322, α261-280, α89-105, and α76-93). H-AChR–immune DQ6 mouse T cells responded moderately (∼20,000 cpm) to peptide α304-322 and did not respond to any other H-AChR α subunit peptides. H-AChR–immune T cells from DQ8×DQ6 F1 mice responded to almost all of the peptides recognized by H-AChR–immune T cells from DQ8 mice.
Figure 6.
T cell epitope mapping on the H-AChR α subunit in DQ6, DQ8, DR3, and DQ8×DQ6 F1 transgenic mice immunized with H-AChR. The transgenic mice were immunized in the footpad with H-AChR (20 μg) in CFA. Seven days later, draining LNCs (popliteal and inguinal) were exposed to each of the overlapping synthetic H-AChR α subunit peptides (40 μg/ml) and H-AChR (0.5 μg/ml), and 3H incorporation was determined. The error bars are SE.
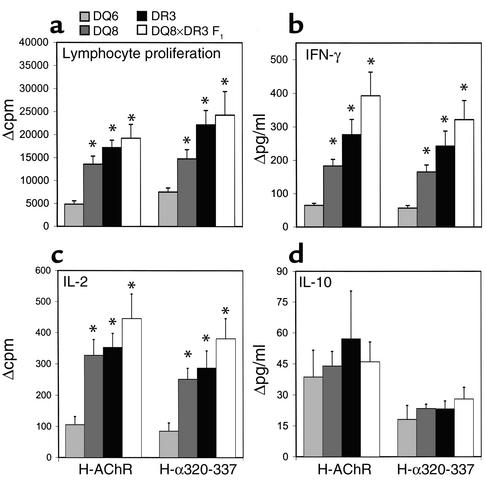
Reduced AChR-specific lymphocyte proliferation and production of IFN-γ and IL-2 in DQ6 mice as compared with DQ8, DR3, and DQ8×DQ6 F1 mice.
Ninety days after the first H-AChR immunization, LNCs from all the transgenic mice were cultured in vitro with H-AChR or peptide H-α320-337, and examined for lymphocyte proliferation and cytokine secretion. H-AChR– and H-α320-337–specific proliferation was significantly lower for LNCs from DQ6 mice than for those from DQ8, DR3, and DQ8×DQ6 F1 mice (Figure 7a). We measured IL-2, IL-10, and IFN-γ in supernatants collected after 48, 72, and 96 hours of culture, respectively. H-AChR– and H-α320-337–specific IFN-γ and IL-2 production was significantly higher in cultures of LNCs from DR3, DQ8, and DQ8×DQ6 F1 mice than in those of LNCs from DQ6 mice (Figure 7, b and c). There were no significant differences in IL-10 responses between different transgenic mice (Figure 7d).
Figure 7.
Reduced H-AChR– and α320-337 peptide–specific lymphocyte proliferative response and IFN-γ and IL-2 secretion in DQ6 mice compared with DQ8, DR3, and DQ8×DR3 F1 mice. At termination of the long-term experiment (day 90), LNCs from DQ6, DQ8, DR3, and DQ8×DR3 F1 mice were cultured in vitro with H-AChR (0.5 μg/ml), peptide α320-337 (40 μg /ml), and PBS, and supernatants collected after 48, 72, and 96 hours were used for IL-2, IL-10, and IFN-γ measurement, respectively. Because of the reduced number of cells, the LNCs from two mice in one group were pooled. For the lymphocyte proliferation, total samples were six DQ6 mice and eight each of DQ8, DR3, and DQ8×DR3 F1 mice; for the supernatant cytokine test, total samples were three DQ6, seven DQ8, five DR3, and five DQ8×DR3 F1 mice. (a) Lymphocyte proliferation is measured in Δcpm (cpm with antigen minus cpm with medium). Mean cpm with medium was: DQ6, 6363; DQ8, 6218; DR3, 6795; DQ8×DR3 F1, 7567. Δcpm for T-AChR was: DQ6, 2152; DQ8, 7213; DR3, 9703; DQ8×DR3 F1, 1046.4. (b and c) Suppressed production of AChR-specific IFN-γ (b) and IL-2 (c) in DQ6 mice compared with DQ8, DR3, and DQ8×DR3 F1 mice. *P < 0.05, Student’s t test. The error bars are SE. Mean values (pg/ml) with medium for IFN-γ were: DQ6, 21.67; DQ8, 22.19; DR3, 36.07; DQ8×DR3 F1, 26.59; and for IL-2 were: DQ6, 27; DQ8, 54.18; DR3, 47.42; DQ8×DR3 F1, 574. (d) IL-10 secretion in supernatant of each strain of transgenic mice. Mean values (pg/ml) with medium were: DQ6, 27; DQ8, 21.4; DR3, 28; DQ8×DR3 F1, 21.79.
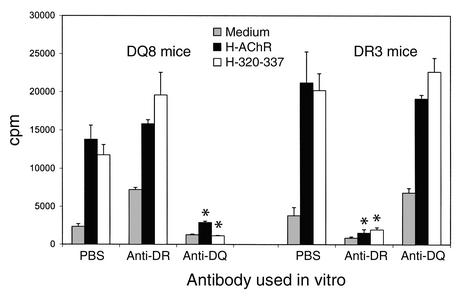
H-AChR– and H-α320-337 peptide–specific lymphocyte responses in DQ8 and DR3 mice are HLA class II–restricted.
DQ8 and DR3 mice were immunized with H-AChR (20 μg per mouse) in CFA. Seven days later, their pooled draining LNCs were stimulated in vitro with H-AChR or H-α320-337, in the presence or absence of anti–HLA-DQ or anti–HLA-DR Ab. Anti–HLA-DQ Ab inhibited the H-AChR– and H-α320-337–induced proliferative response of LNCs from DQ8, but not DR3, mice, while anti–HLA-DR Ab inhibited the H-AChR– and H-α320-337–induced proliferative response of LNCs from DR3, but not DQ8, mice (Figure 8).
Figure 8.
Effect of anti-DR or -DQ Ab on in vitro AChR- and α320-337 peptide–specific lymphocyte proliferation response. Pooled LNCs from four H-AChR–immunized DQ8 mice and four H-AChR–immunized DR3 mice were cultured in vitro with H-AChR (0.5 μg/ml) or H-α320-337 peptide (40 μg/ml), and anti–HLA-DQ or anti–HLA-DR mAb (2.5 μg/ml). *P < 0.05, Student’s t test.
Discussion
Although numerous studies have examined the association of HLA class II gene polymorphism with susceptibility to MG or other autoimmune diseases, the molecular mechanisms by which HLA class II genes influence the development of autoimmune diseases are still obscure. This is because the HLA system is very complex, and the presence of gene clusters and of linkage disequilibrium further complicates this issue. HLA transgenic mice were generated in an attempt to reduce this complexity in a biologically relevant setting. The HLA transgenic mice we used do not express endogenous class II molecules: thus, the only MHC class II gene products involved in antigen presentation in these mice are the human DQ8, DR3, or DQ6 molecules. These HLA class II transgenic mice expressed normal levels of CD4+ and CD8+ cells (25), confirming that the transgenic HLA class II molecules can interact with the mouse CD4 molecule and T cell receptors.
Previous studies used panels of overlapping synthetic peptides spanning the sequence of the different H-AChR subunits, to examine the CD4+ T cell response of PBMCs from MG patients, or H-AChR–specific CD4+ T cell lines derived from MG patients, to identify H-AChR T cell epitopes (29–33, 35–43). Those studies identified a number of sequence regions recognized by CD4+ cells of MG patients, including a few immunodominant epitopes, which were recognized by the majority of MG patients on each human AChR subunit; several of those epitopes were within the H-AChR α subunit. Harcourt et al. reported that peptide 257–269 from H-AChR stimulated cells from six patients and no controls (42). However, MHC class II restriction of this peptide-induced lymphocyte response was not studied. Other studies (44, 45) demonstrated that the AChR sequence regions immunodominant for sensitization of CD4+ cells in MG patients could associate with a variety of purified DR molecules. Those studies demonstrated that the sequence α320-337 was recognized by several MG patients (31, 44, 45), and that this sequence could bind effectively to three of the four DR molecules tested (DR2, DR4, and DR7) (44). T cell lines were generated against the full-length recombinant H-AChR α subunit (41); peptides 75–115 and 138–167 were restricted by DR4, and peptide 309–344 was restricted by DR3 or DR52a (41). However, those studies did not examine the association of any particular dominant H-AChR T cell epitopes with a particular HLA-DQ molecule.
In our current study, we used HLA-DQ8 and DR3 transgenic mice to identify the dominant H-AChR-α subunit T cell epitopes recognized by T cells during the primary and secondary immune response to H-AChR. To reproduce as closely as possible what may occur in the pathogenesis of human MG, we immunized DQ8 and DR3 transgenic mice with intact H-AChR. Therefore, DQ8- and DR3-expressing antigen-presenting cells processed the H-AChR and presented selected peptides to the T cells. DQ8, DR3, DQ8×DR3 F1, and DQ8×DQ6 F1 mice generated anti-AChR Ab and developed clinical EAMG after multiple immunizations with H-AChR. Thus, we have demonstrated here, for the first time to our knowledge that EAMG can be induced in HLA transgenic mice by immunizing with H-AChR. T-AChR–immune T cells of C57BL/6 (I-Ab) mice predominantly respond to epitopes within the sequence region 146–169 of the T-AChR α subunit (20). T cells from HLA-DQ8 and DR3 transgenic mice immunized with T-AChR recognized primarily epitopes within 141–160 and 170–180 of the H-AChR α subunit. However, H-AChR–immune T cells of DQ8 and DR3 transgenic mice responded predominantly to a dominant epitope within residues H-α320-337. These data clearly suggest that T cells of DQ8 mice immunized with T-AChR and H-AChR recognize different epitopes in the H-AChR α subunits. DQ8 mouse T cells responded to most of the peptides recognized by T cells of H-AChR–immune DR3 mice. For H-AChR–sensitized DQ8 mice, peptide H-α320-337 was the dominant epitope. In DR3 mice the anti–H-AChR–immune T cells responded to a number of peptide sequences of the H-AChR α subunit. The DR3 allele has been associated with several autoimmune diseases. The ability of DR3 transgenic mouse T cells to respond to several H-AChR peptides implies that the DR3 molecule can bind numerous epitopes and activate a variety of T cells.
The sequence H-α320-337, which is part of the cytoplasmic domain of the H-AChR, might form a promiscuous epitope (44), recognized by T cells of MG patients with different HLA-DQ or DR alleles, beside the DQ8 and DR3 alleles. The finding that this peptide is frequently recognized by CD4+ cells of MG patients, irrespective of their class II haplotype (30, 31), supports this possibility. Identification of one dominant epitope H-α320-337 for two HLA alleles (DQ8 and DR3) closely linked to MG susceptibility goes beyond the primary mapping of H-AChR T cell epitope in MG patients. Epitopes within this sequence could sensitize CD4+ T cells during the initial priming with H-AChR or with a cross-reactive antigen (i.e., microbial antigen). It is also possible that when the mouse neuromuscular junction AChR is destroyed by Ab binding and complement-mediated lysis, AChR fragments could be processed, and dominant epitope(s) (including α320-337) could be presented to T cells and maintain the autoimmune T cell response to the AChR.
The relative resistance to clinical EAMG of DQ6 mice after immunization with H-AChR and the negative association of the DQ6 allele with human MG could imply that the DQ6 molecule is less efficient in binding and/or presentation of dominant pathogenic T cell epitopes of the H-AChR. It is also possible that individuals bearing the DQ6 allele have reduced T cells potentially reactive to AChR T epitopes. MHC class II molecules influence the peptide-binding characteristics during immune response. HLA-DQ8 and DR3 bind few or more autoantigenic peptides that contribute to MG development, and the DQ6 molecule either does not bind these autoantigenic peptides, or else binds them in an altered binding motif such that different residues could be presented to the T cell receptor.
There was no difference in the anti-AChR IgM Ab response in H-AChR–immunized DQ8, DR3, and F1 mice (all susceptible to EAMG), and the EAMG-resistant DQ6 mice. However, all the serum anti-AChR IgG isotypes we tested (IgG1, IgG2b, and IgG2c) were lower in DQ6 mice than in DQ8, DR3, and F1 mice. This suggests that the B cells of DQ6 mice have antigen receptors specific for mouse AChR in normal numbers, but less capable of mounting a secondary Ab response to the mouse AChR. The overall T cell response to the H-AChR α subunit epitopes leads to a strong production of cytokines (i.e., IL-2 and IFN-γ), which may activate H-AChR–specific B cells in DQ8 and DR3 mice. The defect in switching from anti-AChR IgM to IgG could be due to the reduced T cell responses, and the reduced IFN-γ and IL-2 production that we observed in DQ6 mice after immunization with H-AChR. Also, the resistance of HLA-DQ6 mice to EAMG induced by immunization with T-AChR was associated with a reduced AChR-specific production of IFN-γ, IL-2, and IL-10 (46).
It is of interest that H-AChR–immune T cells of both DQ8 and DR3 mice, which are susceptible to EAMG, responded dominantly to the H-α320-337 peptide. Also, the H-α320-337 peptide response was dominant in DQ8×DQ6 F1 mice. The I-Ab gene was codominant for clinical EAMG susceptibility when mice were immunized with T-AChR (47). Thus, one MG-susceptible HLA-DQ allele in a heterozygote could be sufficient for full expression of MG.
We report several new findings in this paper: (a) H-AChR derived from TE671 muscle-like cell line has been purified by neurotoxin affinity chromatography. (b) EAMG was successfully induced in HLA class II transgenic mice by immunizing with H-AChR. (c) The DQ8 gene conferred susceptibility to clinical EAMG and T cell responsiveness to the dominant peptides H-α320-337, according to a pattern of dominant inheritance. (d) The resistance to EAMG of DQ6 mice was associated with a reduced secondary anti-AChR IgG response, but not with the anti-AChR IgM response. (e) The EAMG resistance of DQ6 mice was associated with a reduced H-AChR–specific production of IFN-γ and IL-2. (f) We have characterized the dominant human class II–restricted H-AChR α subunit T cell epitope H-α320-337 for DQ8 and DR3 transgenic mice.
EAMG can be prevented or suppressed in T-AChR–primed I-Ab mice by injection of high doses of T-α146-162 peptide in incomplete Freund’s adjuvant or by nasal or subcutaneous treatment with solutions of the peptide T-α150-169 (20–23). The H-AChR peptide α320-337, identified here as immunodominant in DR3 and DQ8 transgenic mice, might be used for similar therapeutic tolerance approaches in MG patients.
Acknowledgments
This study was supported by the Muscular Dystrophy Association (P. Christadoss) and the NIH (National Institute of Neurological Disease and Stroke NS-23919; B. Conti-Fine). H. Yang was a Myasthenia Gravis Foundation Osserman/Sosin/McClure postdoctoral fellow and is now an MDA Neuromuscular Disease Research Career Award recipient. M.A. Poussin was a recipient of consecutive postdoctoral fellowship awards from the Association Francaise contre les Myopathies, the Myasthenia Gravis Foundation (Osserman/Sosin/McClure), and the McLaughlin Foundation and is now an MDA Neuromuscular Disease Research Career Award recipient.
References
- 1.Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 2.Engel AG, Lambert EH, Howard FM. Immune complexes (IgG and C3) at the motor end plate in myasthenia gravis: ultra structural and light microscopic localization and electrophysiologic correlations. Mayo Clin Proc. 1977;52:267–280. [PubMed] [Google Scholar]
- 3.Conti-Fine, B.M., Bellone, M., Howard, J.F., Jr., and Protti, M.P. 1997. Myasthenia gravis: the immunobiology of an autoimmune disease. R.G. Landes, editor. Chapman & Hall, Springer. Austin, Texas, USA. 230 pp.
- 4.Christadoss P, Poussin M, Deng C. Animal models of myasthenia gravis. Clin Immunol. 2000;94:75–87. doi: 10.1006/clim.1999.4807. [DOI] [PubMed] [Google Scholar]
- 5.Christadoss P, Lindstrom J, Munro S, Talal N. Muscle acetylcholine receptor loss in murine experimental autoimmune myasthenia gravis: correlated with cellular, humoral and clinical responses. J Neuroimmunol. 1985;8:29–41. doi: 10.1016/s0165-5728(85)80045-x. [DOI] [PubMed] [Google Scholar]
- 6.Christadoss P. C5 gene influences the development of murine myasthenia gravis. J Immunol. 1988;140:2589–2592. [PubMed] [Google Scholar]
- 7.Christadoss P, Lennon VA, Krco CJ, David CS. Genetic control of experimental autoimmune myasthenia gravis in mice. III. Ia molecules mediate cellular immune responsiveness to acetylcholine receptors. J Immunol. 1982;128:1141–1144. [PubMed] [Google Scholar]
- 8.Kaul R, Shenoy M, Goluszko E, Christadoss P. Major histocompatibility complex class II gene disruption prevents experimental autoimmune myasthenia gravis. J Immunol. 1994;152:3152–3157. [PubMed] [Google Scholar]
- 9.Christadoss P, Lindstrom JM, Melvold RW, Talal N. Mutation at I-A beta chain prevents experimental autoimmune myasthenia gravis. Immunogenetics. 1985;21:33–38. doi: 10.1007/BF00372239. [DOI] [PubMed] [Google Scholar]
- 10.Christadoss P, Dauphinee MJ. Immunotherapy for myasthenia gravis: a murine model. J Immunol. 1986;136:2437–2440. [PubMed] [Google Scholar]
- 11.Bell J, et al. HLA-DQ beta-chain polymorphism linked to myasthenia gravis. Lancet. 1986;1:1058–1060. doi: 10.1016/s0140-6736(86)91330-9. [DOI] [PubMed] [Google Scholar]
- 12.Naeim F, et al. Association of HLA-B8, DRw3, and anti-acetylcholine receptor antibodies in myasthenia gravis. Tissue Antigens. 1978;12:381–386. doi: 10.1111/j.1399-0039.1978.tb01347.x. [DOI] [PubMed] [Google Scholar]
- 13.Hjelmstrom P, et al. Polymorphic amino acid domains of the HLA-DQ molecule are associated with disease heterogeneity in myasthenia gravis. J Neuroimmunol. 1996;65:125–131. doi: 10.1016/0165-5728(96)00008-2. [DOI] [PubMed] [Google Scholar]
- 14.Raju R, et al. Polymorphism at the HLA-DQ locus determines susceptibility to experimental autoimmune myasthenia gravis. J Immunol. 1998;160:4169–4174. [PubMed] [Google Scholar]
- 15.Raju R, Spack EG, David CS. Acetylcholine receptor peptide recognition in HLA DR3-transgenic mice: in vivo responses correlate with MHC-peptide binding. J Immunol. 2001;167:1118–1124. doi: 10.4049/jimmunol.167.2.1118. [DOI] [PubMed] [Google Scholar]
- 16.Bellone M, Ostlie N, Lei S, Conti-Tronconi B. Experimental myasthenia gravis in congenic mice. Sequence mapping and H-2 restriction of T helper epitopes on the subunits of Torpedo californicaand murine acetylcholine receptors. Eur J Immunol. 1991;21:2303–2310. doi: 10.1002/eji.1830211003. [DOI] [PubMed] [Google Scholar]
- 17.Bellone M, Ostlie N, Lei S, Wu X-D, Conti-Tronconi B. The I-Abm12 mutation, which confers resistance to experimental myasthenia gravis, drastically affects the epitope repertoire of murine CD4+cells sensitized to nicotinic acetylcholine receptor. J Immunol. 1991;147:1484–1491. [PubMed] [Google Scholar]
- 18.Infante A, Thompson P, Krolik K, Wall K. Determinant selection in murine experimental autoimmune myasthenia gravis: effect of the bm12 mutation on T-cell recognition of acetylcholine receptor epitopes. J Immunol. 1991;146:2977–2982. [PubMed] [Google Scholar]
- 19.Shenoy M, Oshima M, Atassi MZ, Christadoss P. Suppression of experimental autoimmune myasthenia gravis by epitope-specific neonatal tolerance to synthetic region alpha 146-162 of acetylcholine receptor. Clin Immunol Immunopathol. 1993;66:230–238. doi: 10.1006/clin.1993.1030. [DOI] [PubMed] [Google Scholar]
- 20.Wu B, Deng C, Goluszko E, Christadoss P. Tolerance to a dominant T cell epitope in the acetylcholine receptor molecule induces epitope spread and suppresses murine myasthenia gravis. J Immunol. 1997;159:3016–3023. [PubMed] [Google Scholar]
- 21.Karachunski P, Ostlie N, Okita D, Conti-Fine BM. Prevention of experimental myasthenia gravis by nasal administration of synthetic acetylcholine receptor T epitope sequences. J Clin Invest. 1997;100:3027–3035. doi: 10.1172/JCI119857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karachunski PI, Ostlie NS, Okita DK, Garman R, Conti-Fine BM. Subcutaneous administration of T epitope sequences of the acetylcholine receptor prevents experimental myasthenia gravis. J Neuroimmunol. 1999;93:108–121. doi: 10.1016/s0165-5728(98)00208-2. [DOI] [PubMed] [Google Scholar]
- 23.Deng C, Goluszko E, Christadoss P. Fas/Fas ligand pathway, apoptosis, and clonal anergy involved in systemic acetylcholine receptor T cell epitope tolerance. J Immunol. 2001;166:3458–3467. doi: 10.4049/jimmunol.166.5.3458. [DOI] [PubMed] [Google Scholar]
- 24.Abraham RS, et al. NOD background genes influence T cell responses to GAD 65 in HLA DQ8 transgenic mice. Hum Immunol. 1999;60:583–590. doi: 10.1016/s0198-8859(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 25.Abraham RS, Kudva YC, Wilson SB, Strominger JL, David CS. Co-expression of HLA DR3 and DQ8 results in the development of spontaneous insulitis and loss of tolerance to GAD65 in transgenic mice. Diabetes. 2000;49:548–554. doi: 10.2337/diabetes.49.4.548. [DOI] [PubMed] [Google Scholar]
- 26.Wu, B., Goluszko, E., and Christadoss, P. 1997. Experimental autoimmune myasthenia gravis in the mouse. In Current protocols of immunology. Volume 3:15. M. J.E. Coligan, A.M. Kruisheek, D.H. Margulies, E.M. Shevach, and W. Strober, editors. John Wiley & Sons. New York, New York, USA. 8.1–8.16.
- 27.Lindstrom J, Merlie J, Yogeeswaran G. Biochemical properties of acetylcholine receptor subunits from Torpedo californica. Biochemistry. 1979;18:4465–4470. doi: 10.1021/bi00588a003. [DOI] [PubMed] [Google Scholar]
- 28.Houghten RA. General method for the rapid solid phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci USA. 1985;82:7042–7052. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Protti MP, Manfredi AA, Straub C, Howard J, Conti-Tronconi BM. CD4+T cell response to human acetylcholine receptor α subunit in myasthenia gravis. A study with synthetic peptides. J Immunol. 1990;144:1276–1281. [PubMed] [Google Scholar]
- 30.Protti MP, Manfredi AA, Straub C, Howard JF, Conti-Tronconi BM. Immunodominant regions for T-helper sensitization on the human nicotinic receptor α subunit in myasthenia gravis. Proc Natl Acad Sci USA. 1990;87:7792–7796. doi: 10.1073/pnas.87.19.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manfredi AA, Protti MP, Wu XD, Howard JF, Jr, Conti-Tronconi BM. CD4+T-epitope repertoire on the human acetylcholine receptor α subunit in severe myasthenia gravis. A study with synthetic peptides. Neurology. 1992;42:1092–1100. doi: 10.1212/wnl.42.5.1092. [DOI] [PubMed] [Google Scholar]
- 32.Protti MP, et al. Myasthenia gravis. T-epitopes on the δ subunit of human muscle acetylcholine receptor. J Immunol. 1991;146:2253–2261. [PubMed] [Google Scholar]
- 33.Wang ZY, Okita DK, Howard J, Jr, Conti-Fine BM. Th1 epitope repertoire on the alpha subunit of human muscle acetylcholine receptor in myasthenia gravis. Neurology. 1997;48:1643–1653. doi: 10.1212/wnl.48.6.1643. [DOI] [PubMed] [Google Scholar]
- 34.Christadoss P, David CS, Shenoy M, Keve S. EαΚtransgene in B10 mice suppresses the development of myasthenia gravis. Immunogenetics. 1990;31:241–244. doi: 10.1007/BF00204895. [DOI] [PubMed] [Google Scholar]
- 35.Moiola L, Karachunski P, Protti MP, Howard JF, Jr, Conti-Tronconi BM. Epitopes on the beta subunit of human muscle acetylcholine receptor recognized by CD4+ cells of myasthenia gravis patients and healthy subjects. J Clin Invest. 1994;93:1020–1028. doi: 10.1172/JCI117050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Protti MP, et al. Myasthenia gravis: CD4+T epitopes on the embryonic γ subunit of human muscle acetylcholine receptor. J Clin Invest. 1992;90:1558–1567. doi: 10.1172/JCI116024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manfredi AA, Protti MP, Dalton MWM, Howard JF, Jr, Conti-Tronconi BM. T helper cell recognition of muscle acetylcholine receptor in myasthenia gravis: epitopes on the γ and δ subunits. J Clin Invest. 1993;92:1055–1067. doi: 10.1172/JCI116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miola L, Protti MP, Howard JF, Howard JF, Conti-Tronconi BM. Myasthenia gravis: residues of the α and γ subunits of muscle acetylcholine receptor involved in formation of immunodominant CD4+ epitopes. J Immunol. 1994;152:4686–4698. [PubMed] [Google Scholar]
- 39.Wang ZY, Okita DK, Howard JF, Jr, Conti-Fine BM. Th1 epitope repertoire on the α subunit of human muscle acetylcholine receptor in myasthenia gravis. Neurology. 1997;48:1643–1653. doi: 10.1212/wnl.48.6.1643. [DOI] [PubMed] [Google Scholar]
- 40.Wang ZY, Okita D, Howard JF, Jr, Conti-Fine BM. CD4+T cell recognition of the ε subunit of human muscle acetylcholine receptor in myasthenia gravis. J Neuroimmunol. 1998;91:33–42. doi: 10.1016/s0165-5728(98)00146-5. [DOI] [PubMed] [Google Scholar]
- 41.Matsuo H, et al. Peptide-selected T cell lines from myasthenia gravis patients and controls recognize epitopes that are not processed from whole acetylcholine receptor. J Immunol. 1995;155:3683–3692. [PubMed] [Google Scholar]
- 42.Harcourt GC, Sommer N, Rothbard J, Willcox HNA, Newsom-Davis J. A juxta-membrane epitope on the human acetycholine receptor recognized by T cells in myasthenia gravis. J Clin Immunol. 1988;82:1295–1300. doi: 10.1172/JCI113729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill M, et al. Early-onset myasthenia gravis: a recurring T-cell epitope in the adult-specific acetylcholine receptor subunit presented by the susceptibility allele HLA-DR52a. Ann Neurol. 1999;45:224–231. doi: 10.1002/1531-8249(199902)45:2<224::aid-ana13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 44.Manfredi A, Yuen MH, Moiola L, Protti MP, Conti-Tronconi BM. Human acetylcholine receptor presentation in myasthenia gravis: DR restriction of autoimmune T epitopes and binding of synthetic receptor sequences to DR molecules. J Immunol. 1994;152:4165–4174. [PubMed] [Google Scholar]
- 45.Yuen MH, Macklin K, Conti-Fine BM. MHC class II presentation of human acetylcholine receptor in myasthenia gravis. Binding of synthetic gamma subunit sequences to purified DR molecules. J Autoimmun. 1996;9:67–77. doi: 10.1006/jaut.1996.0009. [DOI] [PubMed] [Google Scholar]
- 46.Poussin MA, Goluszko E, David CS, Franco JU, Christadoss P. HLA-DQ6 transgenic mice resistance to experimental autoimmune myasthenia gravis is linked to reduced AChR-specific IFN-γ, IL-2, and IL-10 production. J Autoimmun. 2001;17:175–180. doi: 10.1006/jaut.2001.0541. [DOI] [PubMed] [Google Scholar]
- 47.Christadoss P. Immunogenetics of experimental autoimmune myasthenia gravis. Crit Rev Immunol. 1989;9:247–278. [PubMed] [Google Scholar]