Abstract
Deficiency of the membrane protein FAT/CD36 causes a marked defect in fatty acid uptake by various tissues and is genetically linked to insulin resistance in rats and humans. Here, we examined insulin responsiveness of CD36–/– mice. When fed a diet high in complex carbohydrates and low (5%) in fat, these animals cleared glucose faster than the wild-type. In vivo, uptake of 2-fluorodeoxyglucose by muscle was increased severalfold, and in vitro, insulin responsiveness of glycogenesis by the soleus was enhanced. Null mice had lower glycogen levels in muscle and liver, lower muscle triglyceride levels, and increased liver triglyceride content—all findings consistent with increased insulin-sensitivity. However, when the chow diet was switched to one high in fructose, CD36–/– mice but not wild-type mice developed marked glucose intolerance, hyperinsulinemia, and decreased muscle glucose uptake. High-fat diets impaired glucose tolerance equally in both groups, although CD36 deficiency helped moderate insulin-responsive muscle glucose oxidation. In conclusion, CD36 deficiency enhances insulin responsiveness on a high-starch, low-fat diet. It predisposes to insulin resistance induced by high fructose and partially protects from that induced by high-fat diets. In humans, CD36 deficiency may be an important factor in the metabolic adaptation to diet and in susceptibility to some forms of diet-induced pathology.
Introduction
High blood fatty acids (FAs) are a common feature of insulin-resistant states (1), and raising the level of plasma FAs can induce acute insulin resistance (2). Plasma FAs and insulin sensitivity are negatively correlated (2), and an even stronger negative correlation can be documented with intramuscular triglycerides (TGs) (3, 4). Randle et al. (5) originally stated that excessive muscle FA oxidation induced insulin resistance by inhibiting glucose oxidation. The mechanism proposed (6) involved inactivation of pyruvate dehydrogenase, phosphofructokinase, and hexokinase. However, recent measurements of muscle concentrations of glucose and glucose-6-phosphate using noninvasive nuclear magnetic resonance spectroscopy indicate that the negative FA effect on glucose utilization may be exerted primarily at the level of membrane transport (7). In addition, the reduction in intracellular glucose utilization associated with transport inhibition appears to mostly reflect a decrease in glucose conversion to glycogen rather than in glucose oxidation (8).
A valuable model for the study of the effects of alteration in FA utilization on insulin responsiveness is the recently generated CD36-deficient mouse (9). CD36, also known as fatty acid translocase (FAT) (10), is a multispecific, integral membrane glycoprotein (11, 12) that has been identified as a facilitator of FA uptake. Its function in binding and transport of FA was documented in vitro by affinity labeling with FA derivatives and by cell transfection studies (13). Generation of mice deficient in or overexpressing CD36 established the physiological role of the protein. The CD36-deficient mouse (9, 14) exhibits greater than a 60% decrease of FA uptake and utilization by heart, oxidative skeletal muscle, and adipose tissues. In contrast, mice with muscle CD36 overexpression (15) have enhanced FA oxidation in response to contraction, documenting the rate-limiting role of the transport step.
CD36 deficiency has been genetically linked to insulin resistance in the spontaneously hypertensive rat (SHR), a rodent model of human metabolic syndrome X (16, 17). Supplementing the diet with short- and medium-chain FA, which would not require CD36-facilitated transport, improves SHR hyperinsulinemia and myocardial hypertrophy (18).
In contrast to the findings in the SHR, early data in the CD36-null mouse documented fasting hypoglycemia (9), while muscle CD36 overexpression was associated with hyperglycemia and hyperinsulin-emia (15). In humans, although incidence of CD36 deficiency (19) is highest (6–18.5%) in subpopulations with a high prevalence of diabetes type 2, such as African-Americans (20) and Africans (21), preliminary findings yielded divergent conclusions regarding a possible link with insulin resistance (22, 23). We speculated that the variable results may reflect an effect of environmental conditions, and especially dietary ones. In this respect, it is worth noting that most studies of insulin resistance in the CD36-deficient SHR reported using a high-fructose diet (17, 24). This interpretation would also be in line with the evidence that manifestation of insulin resistance in humans reflects both genetic and dietary influences (25). So we examined the CD36-null mouse for insulin responsiveness and susceptibility to diet-induced insulin resistance. The CD36-null mouse is genetically simpler than the SHR and may provide a better model for the CD36-deficient human. The defect in myocardial FA uptake, consequent to CD36 deficiency, measured in vivo, is similar and approximates 70% in both mice (14) and humans (26), while it is smaller (25%) in the SHR (18).
Methods
Animals.
CD36-null mice and wild-type (WT) control littermates of identical genetic background (93.75% C57BL/6 and 6.25% 129SvJ) (9, 14) were housed in a facility equipped with a 12-hour light cycle and were fed ad libitum a standard chow diet (diet no. 5001; Purina Mills Inc., St. Louis, Missouri, USA), a fructose-rich diet (diet no. 00202; Harlan Teklad, Madison, Wisconsin, USA), or a high-fat diet (diet no. 99012501; Research Diets Inc., New Brunswick, New Jersey, USA). The chow diet contained 50% per weight complex carbohydrate, 22% protein, and 6.5% fat mostly as polyunsaturated FAs. The fructose diet (24) consisted of 60% fructose, 20% protein, and 7% fat as soybean oil. The high-fat diet (27) contained 18.2% sucrose, 33% casein, and 32% safflower oil. Mice were fed safflower and fructose diets for 16 and 12 weeks, respectively, in line with the length of time required to induce insulin resistance, as reported in previous studies (24, 27).
Age- and sex-matched littermates were used for the studies at 14–18 weeks when weights ranged from 20 to 24 g for females and 28 to 35 g for males. All studies were in compliance with the guidelines of the institutional animal care committee.
Analysis of plasma parameters.
Tail-vein blood (about 200 μl) was collected from fasted (16 hours) mice into heparin or EDTA-containing (FA determination) tubes. Plasma FFA was measured using a kit from Wako Chemicals USA Inc. (Richmond, Virginia, USA), TGs and glucose with kits from Sigma-Aldrich (St. Louis, Missouri, USA), and insulin with an RIA kit from Linco Research Inc. (St. Charles, Missouri, USA).
Glucose tolerance test.
Mice fasted for 16 hours were injected intraperitoneally with a 25% glucose solution (1.5 g/kg). Blood (5–10 μl) was collected from the tail prior to and at 10, 20, 30, 45, 60, 90, 120, and 180 minutes after injection. Glucose was measured using a Precision Q.I.D. monitoring system (MediSense; Abbott Laboratories, Abbott Park, Illinois, USA). A similar protocol was used for measuring the response of plasma insulin to the glucose load except that blood (100 μl) was collected at 0, 30, and 60 minutes after injection for insulin and glucose determination.
Uptake and tissue distribution of fluorodeoxyglucose.
Fasted mice were injected in a lateral tail vein with 100 μl of fluorodeoxyglucose (18F-2-FDG) in saline (about 5 μCi, half-life of 110 minutes). For determining decay of blood FDG–specific activity, blood samples (25 μl) were collected and counted at the indicated times after FDG injection, and glucose concentration was measured. The mice were killed after 2 hours, and the tissues were rapidly removed, rinsed with cold saline, and blotted dry. Tissues along with blood samples and an aliquot of the injected solution were counted in an NaI auto–gamma counter. To adjust for the difference in blood glucose between WT and CD36-null mice, specific activity of blood FDG at 2 minutes after injection was used as the 100% value (28, 29).
Tissue FDG at the end of the experiment reflects total uptake, since phosphorylated FDG is trapped intracellularly and not metabolized further. Glucose uptake by each tissue was calculated by dividing tissue 18F-2-FDG counts by the calculated integral of blood specific activity (SA) according to the equation:
| 1 |
where 18F-2-FDG = counts per g tissue at the end of the experiment (2 hours), and SA = counts/μg.
Glycogen and TG content.
Livers, hearts, and hind limbs were harvested from anesthetized mice, freeze-clamped in liquid nitrogen, and stored at –80°C for later analysis. Tissue glycogen was measured as glucose after hydrolysis with KOH (30%) and HCL (0.6 N) (30). TG content was determined enzymatically after lipid extraction (31) as previously described (32). Tissue protein was determined according to the method of Markwell et al. (33).
Glycogenesis and glucose oxidation in incubated soleus muscle.
Rates of glycogenesis in isolated muscles were determined as previously described (34). Extensor digitorum longus (EDL) and soleus muscles were removed intact from anesthetized mice and preincubated (30 minutes) in Krebs-Henseleit buffer (pH 7.4) containing glucose (8 mM) in the absence or presence of maximal stimulating concentrations of insulin (20 mU/ml). Incubations were for 60 minutes in the same fresh buffer that now also contained [U14C]-D-glucose (0.5 μCi per vial). At the end of the incubation, muscles were blotted dry, frozen in liquid nitrogen, and stored at –80°C until analyzed. Glycogen was precipitated using standard procedures (34). Rate of glycogen synthesis from D-glucose was determined from the 14C content of the glycogen pool. Glucose oxidation was monitored from the 14CO2 produced. This was determined by transferring a 0.5-ml aliquot of incubation buffer to a sealed glass vial and acidifying the buffer with 1 M H2SO4. Liberated 14CO2 was captured by a suspended center well containing benzethonium hydroxide. Center wells were placed in scintillation vials and counted.
Statistical analyses.
Data are shown as means ± SE. They were calculated using InStat (GraphPad Software, San Diego, California, USA) and analyzed using two-tailed unpaired t test. Results for the glucose tolerance tests were analyzed with two-factor repeated-measures ANOVA before application of the two-tailed unpaired t tests.
Results
Plasma glucose and insulin and glucose tolerance tests.
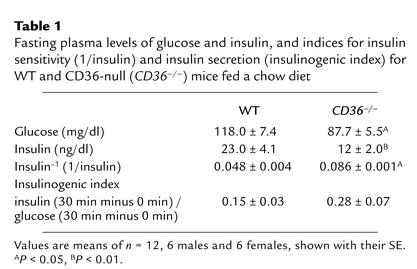
Compared with those in WT mice, fasting glucose and insulin levels were significantly lower in CD36-null mice. Data shown (Table 1) are for both sexes combined. However, the hypoinsulinemia was more pronounced in males than in females (insulin was 54% versus 33% lower, respectively). The index 1/insulin was higher in CD36-null mice, suggesting enhanced insulin sensitivity.
Table 1.
Fasting plasma levels of glucose and insulin, and indices for insulin sensitivity (1/insulin) and insulin secretion (insulinogenic index) for WT and CD36-null (CD36–/–) mice fed a chow diet
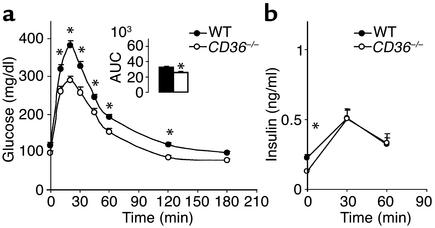
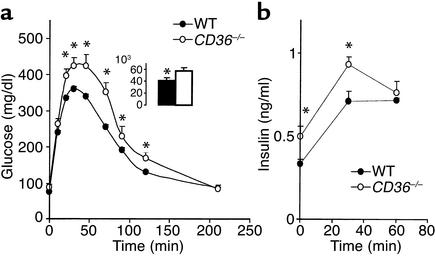
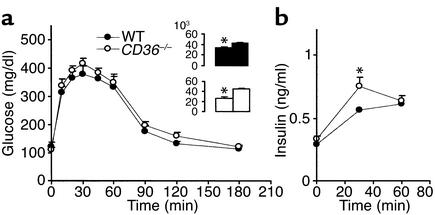
CD36-null mice (males and females) had a significantly enhanced ability to clear an intraperitoneal glucose load (Figure 1a). At 20 minutes after the load, plasma glucose reached a peak concentration, which was about 30% lower in the CD36-null mice. Area under the clearance curve (inset) was 22% lower in null mice as compared with age- and sex-matched WTs (P < 0.001). To determine whether the insulin response to glucose was altered, plasma insulin was measured before and at 30 and 60 minutes after the glucose load. Insulin levels after glucose administration (Figure 1b) and the insulinogenic index (Table 1), which reflects pancreatic β cell function (35, 36), were similar for WT and CD36-null mice.
Figure 1.
Response of blood glucose (a) and insulin (b) to a glucose load in CD36-null (CD36–/–) and WT mice fed a standard chow diet. Twelve-week-old mice fasted for 16 hours were given glucose (1.5 mg/g) intraperitoneally. (a) Blood glucose was measured before and at 10, 20, 30, 45, 60, 120, and 180 minutes after glucose administration. Two-way repeated-measures ANOVA indicated a significant effect of the genotype (P < 0.05). The change of glucose response over time in each genotype (P < 0.05) and the interaction genotype × glucose are also significant (P < 0.05). *Significant differences (t test) between CD36–/– and WT at each time point. P < 0.01 for 20–60 minutes and P < 0.05 for 120 minutes. Inset shows areas under the glucose tolerance curves (AUC) (P < 0.01). (b) Plasma insulin levels were determined before the glucose injection and at 30 and 60 minutes after injection. *Insulin levels at 0 minutes are significantly lower in CD36–/– than in WT (P < 0.05). All data are means ± SEM with n = 12 (6 males and 6 females).
Tissue uptake of glucose in vivo.
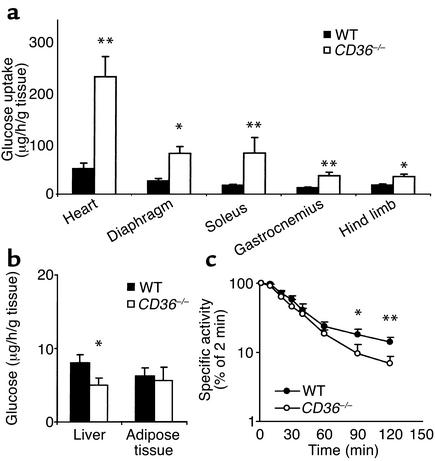
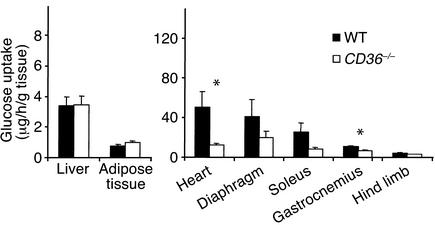
To identify the tissues responsible for the increased clearance of blood glucose, we measured glucose uptake in vivo using radioactive 18F-2-FDG. As shown in Figure 2a, uptake of FDG was increased in hearts (five times), diaphragms (three times), soleus (four times), gastrocnemius (three times), and hind limb muscle (two times) from CD36-null mice as compared with WT mice. Uptake was unaltered in adipose tissue and decreased in liver (Figure 2b). During the experiment, blood glucose level was constant in both WT and CD36-null mice. However, decay of 18F-2-FDG–specific activity in blood (Figure 2c) was faster in CD36-null mice, indicating that more endogenous glucose was being released into the blood to dilute specific activity of the FDG tracer. Since the mice were fasted overnight, the major glucose source would be the liver, suggesting increased hepatic glucose output.
Figure 2.
18F-2-FDG uptake (a and b) and FDG blood clearance (c) in CD36-null and WT mice fed the chow diet. Mice were injected with 5 μCi of 18F-2-FDG in a lateral tail vein. Blood samples were collected at 2, 10, 20, 30, 45, 60, 90, and 120 minutes after injection and were tested for radioactivity and glucose content. At the end of the experiment, tissues were removed, weighed, and counted for 18F-2-FDG radioactivity; uptake rate (a and b) is expressed per gram wet tissue. (c) Decay of FDG-specific activity (cpm/μg), calculated as percent of specific activity at 2 minutes after injection, is shown. Data are means ± SEM; n = 6 per group. *P < 0.05, **P < 0.02.
In a subsequent experiment (data not shown), we tested the response of tissue glucose uptake to exogenous administration of a high dose of insulin (0.5 IU/kg body weight). Insulin produced severalfold increases in 18F-2-FDG uptake in heart, diaphragm, soleus muscle, gastrocnemius, and adipose tissues of WT mice. In CD36-null mice, the insulin-induced increases in FGD uptake were significantly smaller as compared with those in WT, for heart, diaphragm, and soleus. This was in line with the observation that oxidative muscles of CD36-null mice had optimal rates of glucose utilization at the endogenous insulin levels present in the fasted state.
Glucose utilization by incubated muscle in vitro.
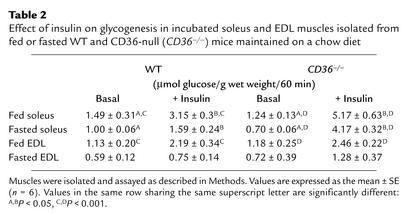
To directly examine insulin sensitivity, in vitro tests were carried out using the isolated soleus, a mostly oxidative muscle; and the EDL, a mostly glycolytic muscle. As shown in Table 2, glycogenesis by the soleus in response to a maximal concentration of insulin was superior for muscles from both fed (+ 316%) and fasted (+ 495%) CD36-null mice as compared with corresponding muscles from fed (+ 111%) and fasted (+ 59%) WT mice. In contrast, there was no significant alteration of the insulin response of the EDL. Glucose oxidation by soleus and EDL muscles from WT and CD36-null mice was similar and responded equally well to insulin (data not shown). Thus, in CD36-null mice, the effect of insulin on glycogenesis in the soleus but not in the EDL was enhanced, while insulin effect on glucose oxidation was not altered.
Table 2.
Effect of insulin on glycogenesis in incubated soleus and EDL muscles isolated from fed or fasted WT and CD36-null (CD36–/–) mice maintained on a chow diet
Muscle glycogen and TG content.
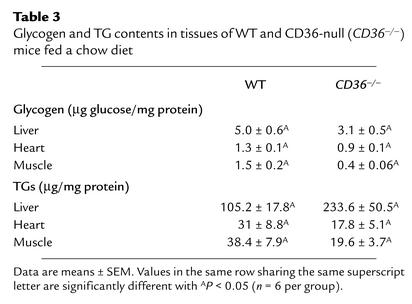
Glycogen and TG contents were measured in heart and hind limb, which are typical of oxidative and glycolytic muscles, respectively. Liver content was determined since it plays an important role in the homeostasis of both plasma glucose and lipids. As shown in Table 3, CD36-null mice had lower glycogen levels in the liver and in heart and hind limb muscles.
Table 3.
Glycogen and TG contents in tissues of WT and CD36-null (CD36–/–) mice fed a chow diet
TG levels were decreased in muscle and heart of CD36-null mice (by 49% and 42%, respectively) but were increased twofold in the liver (Table 3).
Effects of high-fructose and high-fat diets
We next examined whether the high glucose-to-FA utilization ratio created by CD36 deficiency increases susceptibility to metabolic pathology from diets with a high glycemic load while protecting from that induced by diets high in fat.
High-fructose diet.
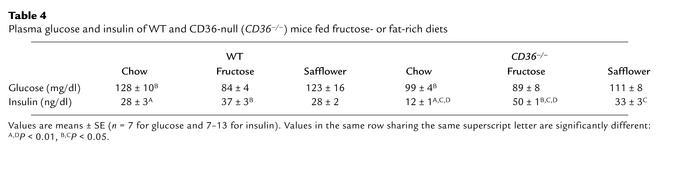
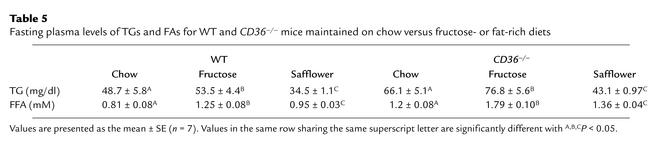
A diet rich in fructose induces a syndrome X–like metabolic phenotype in the SHR but not in the normal control rat (16, 17). In WT and CD36-null mice (Table 4), fructose feeding did not alter blood glucose but it increased blood insulin. The increase was small in WT mice (37 versus 28), while it was more than fourfold for CD36-null mice (50 versus 12). Blood levels of FAs and TGs (Table 5) were increased by the fructose diet in both groups but were higher in null as compared with WT mice on both the chow and fructose diets.
Table 4.
Plasma glucose and insulin of WT and CD36-null (CD36–/–) mice fed fructose- or fat-rich diets
Table 5.
Fasting plasma levels of TGs and FAs for WT and CD36–/– mice maintained on chow versus fructose- or fat-rich diets
Glucose tolerance of CD36-null mice was markedly impaired by the fructose diet, while no effect was observed in WT mice (Figure 3a). Area under the clearance curve (inset) was higher in null than in WT mice fed fructose (P < 0.01). The impairment in glucose tolerance in null mice was significant after 3 weeks on the diet (data not shown), although it was less pronounced than at the time the mice were killed at 12 weeks.
Figure 3.
Response of blood glucose (a) and insulin (b) to a glucose load in CD36-null (CD36–/–) and WT mice fed a fructose-rich diet. Mice were fed a diet containing 60% fructose for 12 weeks. After a 16-hour fast, glucose clearance (a) and plasma insulin (b) were tested in response to a glucose load as described in the legend to Figure 1 and in Methods. Data are means ± SEM (n = 7). (a) Two-way repeated-measures ANOVA indicates that change of glucose response over time in each genotype and the interaction genotype × glucose are significant (P < 0.05). *Significant differences (t test) between CD36–/– and WT at each time point. P < 0.01 for 30, 45, and 60 minutes, and P < 0.05 for 20, 90, and 120 minutes. Inset shows areas under the glucose tolerance curves (P < 0.05). (b) *Insulin levels at 0 and 30 minutes are significantly higher in CD36–/– than in WT (P < 0.05).
CD36-null mice fed fructose, secreted more insulin in response to the glucose load than did WT mice (Figure 3b). Nulls also exhibited peripheral insulin resistance, since FDG uptake by heart and gastrocnemius muscles was significantly lower than uptake by corresponding muscles from WT mice (Figure 4). This contrasted with the situation on the chow diet (Figure 2), in which FDG uptake in null muscles was severalfold higher than in WT muscles. A comparison of FDG uptake in CD36-null hearts on chow (Figure 2) versus fructose (Figure 4) yields values of 240 versus 20 μg/h/g tissue, which represents greater than a 90% drop, while FDG uptake by WT hearts averaged 50 μg/h/g for both diets.
Figure 4.
18F-2-FDG uptake by tissues of CD36-null and WT mice fed a high-fructose diet. 18F-2-FDG (5 μCi) was injected into a lateral tail vein of mice fasted for 16 hours that were maintained on a high-fructose diet for 12 weeks. FDG uptake was determined as described in the legend to Figure 2 and in Methods. Data are means ± SEM (n = 7). *P <0.05.
High-fat diet.
We examined whether CD36 deficiency, which impairs FA utilization by muscle, would protect against peripheral insulin resistance consequent to increased consumption of dietary fat. A diet rich in safflower oil (37, 38) has been shown to be the most effective in impairing glucose tolerance in the C57BL/6J mouse line and was used for these studies. This diet induces peripheral insulin resistance without increasing blood insulin and TG, since it inhibits hepatic lipogenesis and VLDL production (38).
As shown in Table 4, blood levels of glucose and insulin were similar in WT and CD36-null mice fed the high-safflower diet, while TG and FA levels (Table 5) were higher in the CD36-null mice.
Glucose tolerance was impaired by safflower feeding to an equal extent in WT and CD36-null mice (Figure 5a), and the areas under the clearance curves were similar. However, clearance curves were significantly different for each group when compared with those of the mice on the chow diet (P < 0.05). The CD36-null mice fed safflower secreted significantly more insulin than the WT mice at 30 but not at 60 minutes after the glucose load, as shown in Figure 5b.
Figure 5.
Response of blood glucose (a) and insulin (b) to a glucose load in CD36-null (CD36–/–) and WT mice fed a high-fat diet. Mice were fed a diet high in safflower oil for 16 weeks. After a 16-hour fast, glucose clearance (a) and plasma insulin (b) were tested in response to a glucose load as described in the legend to Figure 1 and in Methods. Data are means ± SEM (n = 7). (a) Two-way repeated-measures ANOVA indicates that the interaction genotype × glucose is not significantly different between WT and CD36-null mice. Inset shows that area under the glucose response curve for safflower-fed mice (right bars) was significantly different (P < 0.05) from that for mice fed chow (left bars). Black bars, WT mice; white bars, CD36-null mice. *P <0.05. (b) Insulin response to the glucose load in WT and CD36–/– mice. *Insulin levels at 30 minutes are significantly higher in CD36–/– than in WT (P < 0.05).
Tissue TGs in mice fed fructose or high-fat diets.
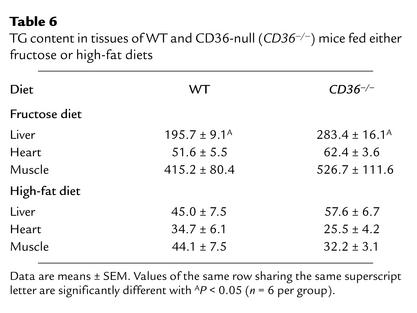
For the high-fructose diet (Table 6), TG levels in livers from CD36-null mice were higher than those in WT livers, while levels in heart and skeletal muscle were similar for both groups. When data are compared with those for mice on the chow diet, TG levels were increased by fructose feeding in all tissues. The relative increase was most marked in muscle (11- and 26-fold for WT and null, respectively).
Table 6.
TG content in tissues of WT and CD36-null (CD36–/–) mice fed either fructose or high-fat diets
For the high-safflower diet (Table 6), TG levels in heart and hind limbs were about 25% lower for CD36-null than for WT mice; however, the differences did not reach statistical significance (n = 6 per group). Levels were similar in the liver. When data are compared with those for mice on the chow diet, liver TGs for both mouse groups were lower on the safflower diet, which has been reported to inhibit hepatic lipogenesis (38).
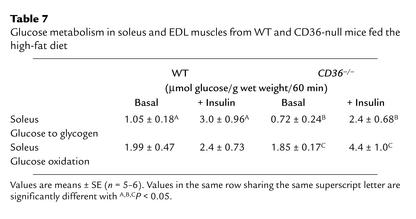
Insulin sensitivity of glucose utilization in muscles of mice fed the safflower diet was examined in the incubated soleus in vitro. As shown in Table 7, glucose incorporation into glycogen exhibited similar responsiveness to a maximal concentration of insulin, when muscles from WT and CD36-null mice are compared. In contrast, glucose oxidation by the soleus was almost unresponsive to insulin in WT muscles, while it was still responsive in CD36-deficient muscles.
Table 7.
Glucose metabolism in soleus and EDL muscles from WT and CD36-null mice fed the high-fat diet
Discussion
FA uptake and insulin sensitivity.
The CD36-deficient mouse, which exhibits defective FA uptake (14, 18) and severely reduced FA oxidation by heart and oxidative muscle, provides a valuable model for examining the link between FA uptake and insulin responsiveness (39).
As shown in this study, the presence of high blood FAs is not sufficient to induce peripheral insulin resistance. The CD36-null mouse maintained on a chow diet has high blood FAs and TGs but is more insulin-sensitive than the WT mouse. The data illustrate the concept that muscle insulin responsiveness is linked to the balance between FA uptake and FA oxidation, with resistance reflecting conditions where uptake exceeds oxidative capacity. In the CD36-null mouse, FA uptake is decreased below the muscle’s capacity to both oxidize and esterify FAs. This is evidenced by large drops in unincorporated intracellular FA (14) as well as in muscle TG levels (Table 3). Our data are consistent with previous findings that deactivation of the PPARα nuclear receptor, which regulates expression of proteins related to muscle FA utilization, is associated with an amelioration of insulin resistance in the apoE-null mouse (40). CD36 expression in muscle is markedly decreased by deactivation of PPARα, which would be expected to impair peripheral FA uptake.
The enhanced insulin sensitivity of the CD36-null mouse contradicts the previously documented linkage of CD36 deficiency to insulin resistance in the SHR (16, 24). However, there is an important difference between the CD36-null mouse and the SHR with respect to muscle FA metabolism. In the SHR, there is evidence to indicate that, despite CD36 deficiency, muscle FA uptake exceeds oxidative capacity. First, the defect in FA uptake by SHR muscle is modest (25% versus 70% in the CD36-null mouse). Second, a fivefold rise in unincorporated cellular FAs was measured in SHR muscle (18), while a 50% decrease is observed in the CD36-null mouse muscle (14). Third, net FA incorporation into TGs is unchanged in SHR muscle (18), which contrasts with a 50% drop in the mouse (14). In line with this, there is a small rise in muscle TG levels in the SHR (41), as opposed to the almost 50% drop in muscle TG in the CD36-null mouse (this study). The biochemical mechanism for these differences still has to be determined. One reason may be that a smaller proportion of exogenous FAs is cycled into TGs before oxidation in rat versus mouse muscle (42). So in the rat, defective uptake would impact FA oxidation more than it would impact FA esterification. In addition, CD36 may facilitate a smaller fraction of FA uptake by rat than by mouse muscle (18).
A significant observation in the present study is that the WT mouse fed high fructose does not exhibit glucose intolerance and decreased muscle glucose utilization despite markedly increased muscle TGs. This supports the interpretation that muscle TGs correlate positively with insulin resistance (43, 44) only when levels reflect excess FA uptake relative to oxidation and that resistance is linked to the accumulation of FA metabolites, possibly FA-acyl-CoA (44).
In the CD36-null mouse, the defect in FA uptake results in strongly enhanced glucose uptake and incorporation into glycogen by muscle. This is a mirror image of the findings in humans, where the major effects of increased FA supply are reductions in muscle glucose uptake and glycogenesis (45, 46). The important role of oxidative muscle in mediating the effects on overall insulin responsiveness is highlighted by the data, since the enhanced insulin sensitivity was primarily limited to oxidative muscles, where the FA defect is most pronounced.
Finally, it is important to note the effect of muscle FA uptake on liver metabolism. Although CD36 expression in the liver is normally low (10), the impact of the deficiency on liver metabolism is significant. Consequent to the defect in peripheral FA utilization, FA flux to the liver is increased. As a result, hepatic FA uptake (14) and FA incorporation into TGs and cholesteryl ester (C. Coburn et al., unpublished observations) are enhanced, increasing hepatic TG content and production of VLDLs (9). Livers of CD36-null mice also had decreased glycogen content, reflecting increased hepatic glucose output and the need to supply more glucose to heart and muscle tissues.
Defect in FA uptake and adaptation to high-fructose and high-fat diets.
Epidemiological data indicate that the Western diet is associated with a higher incidence of type 2 diabetes (recently reviewed in ref. 47). This diet is characterized by a high content of saturated fat and of simple, rapidly absorbed carbohydrates. There is evidence to support a negative effect of both components on insulin responsiveness of glucose utilization. Diets high in fat induce peripheral insulin resistance, in part by promoting obesity (47, 48). Similarly, diets rich in simple sugars, such as sucrose or fructose, which are rapidly absorbed and which have a high glycemic index, when given in high quantities (high glycemic load) promote the glucose intolerance and dyslipidemia characteristic of the metabolic syndrome X (47, 49–51). It has been well documented that humans differ in their susceptibility to the diabetogenic effects of both dietary fat and carbohydrates (47, 52), resulting in variability of the reported data. The findings in this study document how alterations in one gene, in this case CD36, differentially modulate the metabolic adaptation to various diets and the susceptibility to diet-induced pathology.
The high susceptibility of the CD36-null mouse to the diabetogenic effect of a high-fructose diet is similar to that previously documented in the SHR (24). In control rats (53, 54) and mice (this study), high-fructose diets produce no change in peripheral insulin responsiveness. In contrast, the SHR strain and the CD36-null mouse are very sensitive to the effect of a high-fructose diet and develop hyperinsulinemia and insulin resistance. The SHR fed fructose has been widely studied as a valid model of human syndrome X. In healthy human volunteers, short-term feeding of a high-fructose diet is not associated with clear symptoms of insulin resistance (25, 55, 56). However, if our findings could be extrapolated to humans, they would suggest that CD36-deficient humans may be at a high risk of developing insulin resistance from diets high in simple sugars like fructose and sucrose.
CD36 deficiency would be expected to protect against the diabetogenic effects of a high-fat diet, since it impairs peripheral FA utilization. In this respect, deactivation of PPARα, which would downregulate muscle CD36, protects from insulin resistance induced by a Western diet (57). Despite the impaired glucose tolerance observed in CD36-null mice fed high safflower, there was partial protection, as shown with in vitro soleus incubations, where glucose oxidation remained responsive to insulin in CD36-deficient muscles. In addition, preliminary studies of glucose metabolism under hyperinsulinemic clamp conditions indicate a partially protective effect of CD36 defi-ciency with respect to insulin responsiveness of he-patic glucose output and of muscle glucose transport (J. Kim et al., unpublished observations). More studies of the response of CD36-deficient animals to high-fat feeding will be needed, as they should help dissect the respective roles of the liver and muscle in the etiology of insulin resistance.
In summary, based on our data, CD36-deficient humans would be predicted to exhibit enhanced or impaired insulin resistance depending on the diet consumed. They could be particularly susceptible to metabolic pathology from diets with a high glycemic load, which have been positively correlated to incidence of non–insulin-dependent diabetes (58). In contrast, they may be less susceptible to the diabetogenic effects of diets high in fat. It also remains to be determined whether high CD36 expression levels in muscle would increase susceptibility to insulin resistance from high-fat diets. Since incidence of CD36 deficiency in humans ranges from 0.3% to 18.5% depending on the subpopulation (20, 21), it would be important to examine the link between functional levels of CD36 and insulin responsiveness in humans. However, the influence of environmental factors is likely to make such a study very complex. In addition to diet, lifestyle may be important, since contraction influences muscle FA uptake and sarcolemmal levels of CD36 (59, 60). The difficulty environmental interactions create in studies of population genetics has been illustrated recently by work on the LDL and PPARγ genes (61, 62).
Acknowledgments
We thank the PET scanning facility at North Shore University Hospital (Manhasset, New York, USA) and Ralph Matacchieri in particular for preparation and donation of the fluorodeoxyglucose. This work was supported by American Heart Association Fellow-ship AHA0020639T (to T. Hajri) and NIH grant RO1-DK33301 (to N.A. Abumrad). The work was also supported by a grant from the Canadian Institutes of Health Research to A. Bonen.
References
- 1.Unger, R.H., and Foster, D.W. 1998. Diabetes mellitus. In Williams textbook of endocrinology. 9th edition. J.D. Wilson, D.W. Foster, H.M. Kronenberg, and P.R. Larson, editors. W.B. Saunders Inc. Philadelphia, Pennsylvania, USA. 973–1059.
- 2.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes. 1997;46:1001–1009. doi: 10.2337/diab.46.6.1001. [DOI] [PubMed] [Google Scholar]
- 3.Pan DA, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 4.Krssak M, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 5.Randle PJ, Newsholme EA, Garland PB. Regulation of glucose uptake by muscle. VIII. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964;93:652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev. 1998;14:263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Febbraio M, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 10.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- 11.Tandon NN, Kralisz U, Jamieson GA. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989;264:7576–7583. [PubMed] [Google Scholar]
- 12.Greenwalt DE, et al. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992;80:1105–1115. [PubMed] [Google Scholar]
- 13.Harmon CM, Abumrad NA. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J Membr Biol. 1993;133:43–49. doi: 10.1007/BF00231876. [DOI] [PubMed] [Google Scholar]
- 14.Coburn CT, et al. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahimi A, et al. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem. 1999;274:26761–26766. doi: 10.1074/jbc.274.38.26761. [DOI] [PubMed] [Google Scholar]
- 16.Aitman TJ, et al. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet. 1999;21:76–83. doi: 10.1038/5013. [DOI] [PubMed] [Google Scholar]
- 17.Pravenec M, et al. Transgenic rescue of defective Cd36 ameliorates insulin resistance in spontaneously hypertensive rats. Nat Genet. 2001;27:156–158. doi: 10.1038/84777. [DOI] [PubMed] [Google Scholar]
- 18.Hajri T, et al. Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. J Biol Chem. 2001;276:23661–23666. doi: 10.1074/jbc.M100942200. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto N, et al. A platelet membrane glycoprotein (GP) deficiency in healthy blood donors: Naka- platelets lack detectable GPIV (CD36) Blood. 1990;76:1698–1703. [PubMed] [Google Scholar]
- 20.Curtis BR, Aster RH. Incidence of the Nak(a)-negative platelet phenotype in African Americans is similar to that of Asians. Transfusion. 1996;36:331–334. doi: 10.1046/j.1537-2995.1996.36496226147.x. [DOI] [PubMed] [Google Scholar]
- 21.Aitman TJ, et al. Malaria susceptibility and CD36 mutation. Nature. 2000;405:1015–1016. doi: 10.1038/35016636. [DOI] [PubMed] [Google Scholar]
- 22.Yanai H, Chiba H, Morimoto M, Jamieson GA, Matsuno K. Type I CD36 deficiency in humans is not associated with insulin resistance syndrome. Thromb Haemost. 2000;83:786. [PubMed] [Google Scholar]
- 23.Miyaoka K, et al. CD36 deficiency associated with insulin resistance. Lancet. 2001;357:686–687. doi: 10.1016/s0140-6736(00)04138-6. [DOI] [PubMed] [Google Scholar]
- 24.Pravenec M, et al. Genetics of Cd36 and the clustering of multiple cardiovascular risk factors in spontaneous hypertension. J Clin Invest. 1999;103:1651–1657. doi: 10.1172/JCI6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bessesen DH. The role of carbohydrates in insulin resistance. J Nutr. 2001;131(Suppl. 10):2782S–2786S. doi: 10.1093/jn/131.10.2782S. [DOI] [PubMed] [Google Scholar]
- 26.Fukuchi K, et al. Enhanced myocardial glucose use in patients with a deficiency in long-chain fatty acid transport (CD36 deficiency) J Nucl Med. 1999;40:239–243. [PubMed] [Google Scholar]
- 27.Ikemoto S, et al. High fat diet-induced hyperglycemia: prevention by low level expression of a glucose transporter (GLUT4) minigene in transgenic mice. Proc Natl Acad Sci USA. 1995;92:3096–3099. doi: 10.1073/pnas.92.8.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacquez JA. Theory of production rate calculations in steady and non-steady states and its application to glucose metabolism. Am J Physiol. 1992;262:E779–E790. doi: 10.1152/ajpendo.1992.262.6.E779. [DOI] [PubMed] [Google Scholar]
- 29.Rajkumar K, Krsek M, Dheen ST, Murphy LJ. Impaired glucose homeostasis in insulin-like growth factor binding protein-1 transgenic mice. J Clin Invest. 1996;98:1818–1825. doi: 10.1172/JCI118982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passonneau JV, Lauderdale VR. A comparison of three methods of glycogen measurement in tissues. Anal Biochem. 1974;60:405–412. doi: 10.1016/0003-2697(74)90248-6. [DOI] [PubMed] [Google Scholar]
- 31.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 32.Hajri T, Khosla P, Pronczuk A, Hayes KC. Myristic acid-rich fat raises plasma LDL by stimulating LDL production without affecting fractional clearance in gerbils fed a cholesterol-free diet. J Nutr. 1998;128:477–484. doi: 10.1093/jn/128.3.477. [DOI] [PubMed] [Google Scholar]
- 33.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 34.Bonen A, McDermott JC, Tan MH. Glycogenesis and glyconeogenesis in skeletal muscle: effects of pH and hormones. Am J Physiol. 1990;258:E693–E700. doi: 10.1152/ajpendo.1990.258.4.E693. [DOI] [PubMed] [Google Scholar]
- 35.Salehi A, Chen D, Kanson R, Nordin G, Lundquist I. Gastrectomy induces impaired insulin and glucagon secretion: evidence for a gastro-insular axis in mice. J Physiol. 1999;514:579–591. doi: 10.1111/j.1469-7793.1999.579ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cretti A, et al. Assessment of beta-cell function during the oral glucose tolerance test by a minimal model of insulin secretion. Eur J Clin Invest. 2001;31:405–416. doi: 10.1046/j.1365-2362.2001.00827.x. [DOI] [PubMed] [Google Scholar]
- 37.Kubota N, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 38.Moir AM, Park BS, Zammit VA. Quantification in vivo of the effects of different types of dietary fat on the loci of control involved in hepatic triacylglycerol secretion. Biochem J. 1995;308:537–542. doi: 10.1042/bj3080537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roden M, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tordjman K, et al. PPARα deficiency reduces insulin resistance and atherosclerosis in apoE-null mice. J Clin Invest. 2001;107:1025–1034. doi: 10.1172/JCI11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaPier TL, Rodnick KJ. Changes in cardiac energy metabolism during early development of female SHR. Am J Hypertens. 2000;13:1074–1081. doi: 10.1016/s0895-7061(00)00297-1. [DOI] [PubMed] [Google Scholar]
- 42.Christe ME, Rodgers RL. Altered glucose and fatty acid oxidation in hearts of the spontaneously hypertensive rat. J Mol Cell Cardiol. 1994;26:1371–1375. doi: 10.1006/jmcc.1994.1155. [DOI] [PubMed] [Google Scholar]
- 43.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 44.Kim JK, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boden G, Jadali F. Effects of lipid on basal carbohydrate metabolism in normal men. Diabetes. 1991;40:686–692. doi: 10.2337/diab.40.6.686. [DOI] [PubMed] [Google Scholar]
- 46.Kruszynska YT, Mulford MI, Yu JG, Armstrong DA, Olefsky JM. Effects of nonesterified fatty acids on glucose metabolism after glucose ingestion. Diabetes. 1997;46:1586–1593. doi: 10.2337/diacare.46.10.1586. [DOI] [PubMed] [Google Scholar]
- 47.Lichtenstein AH, Schwab US. Relationship of dietary fat to glucose metabolism. Atherosclerosis. 2000;150:227–243. doi: 10.1016/s0021-9150(99)00504-3. [DOI] [PubMed] [Google Scholar]
- 48.Ludvik B, Nolan JJ, Baloga J, Sacks D, Olefsky J. Effect of obesity on insulin resistance in normal subjects and patients with NIDDM. Diabetes. 1995;44:1121–1125. doi: 10.2337/diab.44.9.1121. [DOI] [PubMed] [Google Scholar]
- 49.Liu S, Manson JE. Dietary carbohydrates, physical inactivity, obesity, and the ‘metabolic syndrome’ as predictors of coronary heart disease. Curr Opin Lipidol. 2001;12:395–404. doi: 10.1097/00041433-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 50.McLaughlin T, Abbasi F, Lamendola C, Yeni-Komshian H, Reaven G. Carbohydrate-induced hypertriglyceridemia: an insight into the link between plasma insulin and triglyceride concentrations. J Clin Endocrinol Metab. 2000;85:3085–3088. doi: 10.1210/jcem.85.9.6838. [DOI] [PubMed] [Google Scholar]
- 51.Mathers JC, Daly ME. Dietary carbohydrates and insulin sensitivity. Curr Opin Clin Nutr Metab Care. 1998;1:553–557. doi: 10.1097/00075197-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Daly ME, Vale C, Walker M, Alberti KG, Mathers JC. Dietary carbohydrates and insulin sensitivity: a review of the evidence and clinical implications. Am J Clin Nutr. 1997;66:1072–1085. doi: 10.1093/ajcn/66.5.1072. [DOI] [PubMed] [Google Scholar]
- 53.Pagliassotti MJ, et al. Tissue oxidative capacity, fuel stores and skeletal muscle fatty acid composition in obesity-prone and obesity-resistant rats. Obes Res. 1995;3:459–464. doi: 10.1002/j.1550-8528.1995.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 54.Thresher JS, Podolin DA, Wei Y, Mazzeo RS, Pagliassotti MJ. Comparison of the effects of sucrose and fructose on insulin action and glucose tolerance. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1334–R1340. doi: 10.1152/ajpregu.2000.279.4.R1334. [DOI] [PubMed] [Google Scholar]
- 55.Petersen KF, Laurent D, Yu C, Cline GW, Shulman GI. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes. 2001;50:1263–1268. doi: 10.2337/diabetes.50.6.1263. [DOI] [PubMed] [Google Scholar]
- 56.Moore MC, Davis SN, Mann SL, Cherrington AD. Acute fructose administration improves oral glucose tolerance in adults with type 2 diabetes. Diabetes Care. 2001;24:1882–1887. doi: 10.2337/diacare.24.11.1882. [DOI] [PubMed] [Google Scholar]
- 57.Guerre-Millo M, et al. PPAR-alpha-null mice are protected from high-fat diet-induced insulin resistance. Diabetes. 2001;50:2809–2814. doi: 10.2337/diabetes.50.12.2809. [DOI] [PubMed] [Google Scholar]
- 58.Salmeron J, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 59.Bonen A, Dyck DJ, Ibrahimi A, Abumrad NA. Muscle contractile activity increases fatty acid metabolism and transport and FAT/CD36. Am J Physiol. 1999;276:E642–E649. doi: 10.1152/ajpendo.1999.276.4.E642. [DOI] [PubMed] [Google Scholar]
- 60.Luiken JJ, Willems J, van der Vusse GJ, Glatz JF. Electrostimulation enhances FAT/CD36-mediated long-chain fatty acid uptake by isolated rat cardiac myocytes. Am J Physiol Endocrinol Metab. 2001;281:E704–E712. doi: 10.1152/ajpendo.2001.281.4.E704. [DOI] [PubMed] [Google Scholar]
- 61.Luan J, et al. Evidence for gene-nutrient interaction at the PPARgamma locus. Diabetes. 2001;50:686–689. doi: 10.2337/diabetes.50.3.686. [DOI] [PubMed] [Google Scholar]
- 62.Krauss RM. Genetic recipes for heart-healthy diets. Am J Clin Nutr. 2000;71:668–669. doi: 10.1093/ajcn/71.3.668. [DOI] [PubMed] [Google Scholar]