Abstract
To understand the relationship between host antigen-presenting cells (APCs) and donor T cells in initiating graft-versus-host disease (GVHD), we followed the fate of host dendritic cells (DCs) in irradiated C57BL/6 (B6) recipient mice and the interaction of these cells with minor histocompatibility antigen- (miHA-) mismatched CD8+ T cells from C3H.SW donors. Host CD11c+ DCs were rapidly activated and aggregated in the T cell areas of the spleen within 6 hours of lethal irradiation. By 5 days after irradiation, <1% of host DCs were detectable, but the activated donor CD8+ T cells had already undergone as many as seven divisions. Thus, proliferation of donor CD8+ T cells preceded the disappearance of host DCs. When C3H.SW donor CD8+ T cells were primed in vivo in irradiated B6 mice or ex vivo by host CD11c+ DCs for 24–36 hours, they were able to proliferate and differentiate into IFN-γ–producing cells in β2-microglobulin–deficient (β2m–/–) B6 recipients and to mediate acute GVHD in β2m–/– → B6 chimeric mice. These results indicate that, although host DCs disappear rapidly after allogeneic bone marrow transplantation, they prime donor T cells before their disappearance and play a critical role in triggering donor CD8+ T cell–mediated GVHD.
Introduction
Allogeneic bone marrow transplantation (allo-BMT) has had a major impact on the treatment of hematologic malignancies, BM failures, and inherited hematopoietic disorders, and is finding new application in the treatment of nonhematologic diseases as well. However, graft-versus-host disease (GVHD) is still the major complication after allo-BMT, producing multiple-organ damage, immune deficiency, and infection, such that 10–50% of patients who receive MHC-matched sibling or unrelated donor transplants die from complications of the transplant procedure (1, 2). In these patients, GVHD is mediated by donor T cells that recognize minor histocompatibility antigens (miHAs), which are peptides derived from intracellular proteins that distinguish host from donor (3–6). Following the presentation of miHAs by antigen-presenting cells (APCs), donor CD4+ and CD8+ T cells are triggered by miHAs bound to MHC class I and class II antigens, respectively, to induce acute GVHD (1, 2, 7). In mouse models of human allo-BMT, both CD4+ and CD8+ T cells contribute to mediate GVHD (7–9). However, while CD4+ T cells can cause lethal GVHD in only a few H-2–matched, miHA-mismatched mouse strain combinations, donor CD8+ T cells can induce lethal GVHD in most strain combinations (8, 9). This suggests that CD8+ T cells are the most important T cell subset contributing to miHA-initiated GVHD.
T cell immune responses are initiated by APCs (10, 11). While donor-derived and residual host APCs are both present following allo-BMT, we recently found that host APCs are required for CD8+ T cell–dependent GVHD in a murine model (12). In these studies, infusion of C3H.SW donor BM and CD8+ T cells failed to induce acute GVHD in miHA-mismatched β2-microglobulin gene–deficient (β2m–/–) → C57BL/6 (B6) chimeric mice, a model in which hematopoietic cells of B6 mice, including APCs, were replaced by β2m–/– mouse–derived BM cells (12). The failure of these β2m–/– → B6 chimeric recipients of C3H.SW donor BM and CD8+ T cells to develop acute GVHD indicates that donor-derived APCs are insufficient to induce acute GVHD, and therefore that T cell–host APC interactions are essential for triggering the induction of acute GVHD.
Several cell types can function as APCs, including dendritic cells (DCs), macrophages, B cells, and nonhematopoietic cells (10, 11, 13, 14). However, it remains unclear how host APCs initiate donor CD8+ T cell–mediated acute GVHD in this miHA-mismatched murine model. Since DCs are the most potent APCs specialized for the initiation of primary T cell immunity (10, 11), understanding the relationship between DC activation, T cell–DC interaction, and T cell expansion following allo-BMT will be helpful for further elucidating the pathophysiology of acute GVHD at the cellular and molecular levels.
In this study, we explored the fate of host DCs and their role in activating donor CD8+ T cells following total body irradiation (TBI) and allo-BMT. The results show that the activation of host DCs and donor T cells occur extremely early in transplant recipients following irradiation and cell transplantation. Host DCs are able to activate donor CD8+ T cells within 24 hours, before they themselves disappear as the result of allo-BMT conditioning. These findings point to the immediate allo-BMT period as the key window for the induction, and therefore possible prevention, of acute GVHD.
Methods
Mice.
B6 (H-2b, CD45.2+) or B6/SJL (H-2b, CD45.1+) recipient mice, miHA-mismatched C3H.SW (H-2b, CD45.2+, and Ly9.1+) donor mice, β2m–/– B6 mice (H-2b, CD45.2+), and BALB/c mice (H-2d) were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA) and maintained in sterile conditions. From 2 days before irradiation until 3 weeks after transplant, the drinking water of BMT recipients was supplemented with neomycin sulfate and polymyxin B (Sigma Aldrich, St. Louis, Missouri, USA).
Ab’s.
The primary Ab’s used for cell immunofluorescent and immunohistochemical staining and cell separation, including anti-CD3 (clone 145-2C11), anti-CD4 (clone GK1.5), anti-CD8 (clone RM4-5), biotinylated anti–mouse IFN-γ, biotinylated anti-CD11b (clone M1/70), biotinylated anti-CD11c (clone HL3), biotinylated anti-NK1.1, and biotinylated anti-B220, were obtained from Pharmingen (San Diego, California, USA). FITC–anti-CD11a, FITC–anti-CD40, FITC–anti-CD86, FITC–anti-Ia, FITC, phycoerythrin-conjugated (PE-conjugated) streptavidin, PE–anti-CD25, PE–anti-CD62L, PE–anti-CD69, PE–anti-CD11c, Cychrome–anti-CD8, and Cychrome-streptavidin were also obtained from Pharmingen. All anti-CD4, anti-CD8, anti-B220, anti-CD11b, and anti-CD11c Ab’s conjugated with microbeads and the streptavidin conjugated with microbeads were purchased from Miltenyi Biotech (Auburn, California, USA).
Cell preparations.
Donor BM cells were prepared from C3H.SW mice as previously described (12). T cell–depleted BM (T–BM) cells were further prepared using anti-CD4 and anti-CD8 Ab’s conjugated with magnetic microbeads. Donor CD8+ T cells were purified from C3H.SW mice by one of two protocols. In the first, CD8+ T cells were positively selected from spleens and lymph nodes of C3H.SW mice with anti-CD8+ Ab conjugated with magnetic microbeads. Alternatively, CD8+ T cells were negatively selected by depletion of CD11b, NK1.1, B220, and CD4 cells using magnetic cell sorting (MiniMACS; Miltenyi Biotech) (12, 15). Since murine DCs express CD8 antigen (16), CD11c+ cells were also completely removed using anti-CD11c Ab conjugated with microbeads before purifying donor CD8+ T cells. The purity of isolated donor CD8+ T cells was always more than 95%, as reanalyzed by flow cytometry.
CD11c+ DCs were isolated from B6 or C3H.SW splenocytes by magnetic cell sorting as previously described (15). The purity of isolated CD11c+ DCs was more than 90% as analyzed by flow cytometry. In some experiments, purified DCs were cultured ex vivo in Iscove’s modified Dulbecco’s medium containing 10% FCS and GM-CSF (R&D Systems Inc., Minneapolis, Minnesota, USA) in 96-well plates at a cell concentration of 2 × 105 cells/well. The supernatants were collected at 48 hours for assessing the secretion of IL-12 as previously described (15).
Carboxyl fluorescein succinimidyl ester labeling.
C3H.SW CD8+ T cells were resuspended at a concentration of 1 × 107 cells/ml in 2.5% FBS in PBS. Carboxyl fluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, Oregon, USA) was added to a final concentration of 5 μM. Labeling was quenched with ice-cold PBS after 15 minutes of incubation at 37°C. Cells were washed twice in PBS and resuspended in PBS for transplantation in vivo or in medium for in vitro culture.
Isolation of hepatic lymphocytes.
Hepatic lymphocytes were isolated as previously described (17). Briefly, livers from B6 mice were minced and suspended in PBS containing 1 mM EDTA. Percoll was added to the cell suspension (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA) to a final concentration of 33%. After centrifugation for 30 minutes at room temperature, hepatic lymphocytes were collected from the bottom and contaminating red blood cells were lysed.
BMT and CD8+ T cell transplantation.
Mice underwent allo-BMT as previously described (12). Briefly, normal B6 and β2m–/– B6 recipients received 9.5 Gy TBI administered in two treatments from a 137Cs source. C3H.SW CD8+ T cells (2 × 106 to 3 × 106), with or without 7 × 106 C3H.SW T–BM cells, were transplanted into the recipients by tail vein injection (five to six mice per group per experiment) immediately after irradiation. Recipient mice were weighed twice weekly and were monitored for acute GVHD and for survival. The clinical grading for the cutaneous inflammation of GVHD was followed according to established criteria (2, 18). In some experiments, β2m–/– → B6 chimeric mice were irradiated with 7.5 Gy and used as recipients. To create β2m–/– → B6 chimeric mice, 1 × 107 BM cells derived from β2m–/– B6 mice were transplanted into lethally irradiated B6 mice as previously described (12). These β2m–/– → B6 chimeric mice were maintained for 4 months before subsequent use as recipients of allo-BMT. By this time, more than 96% of hematopoietic cells from BM, lymph node, and spleen were negative for MHC class I antigens (12).
Cytokine assays.
IL-12 (p40) in the serum and supernatants of DC cultures was measured by ELISA according to the manufacturer’s instructions (BioSource International, Camarillo, California, USA). Intracellular IFN-γ in donor T cells was performed as previously described with slight modification (19). Briefly, splenocytes and hepatic lymphocytes were cocultured with MC57SV cells (H-2Db, a cell line derived from B6 mice) irradiated with 30 Gy in the Nunc MicroWell plate precoated with anti-CD3 Ab for 16 hours. GolgiStop (Pharmingen) was added for the last 6 hours of culture. Cells were collected and stained with Cychrome–anti-CD8 Ab with or without FITC–anti-CD11a. After fixation and permeabilization using the Cytofix/Cytoperm Plus Kit (Pharmingen), cells were stained with biotinylated mouse anti–IFN-γ followed by PE-conjugated streptavidin, and then analyzed by flow cytometry.
Mixed lymphocyte reaction.
After irradiation with 15 Gy, the indicated splenic DCs from B6 mice were cocultured with purified BALB/c CD4+ T cells in 96-well MicroWell tissue-culture plates (Nunc A/S, Roskilde, Denmark). After incubation for 4 days, cell proliferation was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma Aldrich) assay as previously described (20).
Flow cytometry analysis.
Immunofluorescence analyses were performed as previously described (21). In two-color analyses, splenic DCs were stained with FITC-conjugated anti-CD86, anti-Ia, and PE-conjugated anti-CD11c Ab’s. In other experiments, CFSE-labeled CD8+ T cells were further stained with Cychrome-conjugated anti-CD8 coupled with PE-conjugated anti-CD25, anti-CD69, and anti-CD62L Ab’s. Cells were analyzed by FACScan (Becton Dickinson Immunocytometry Systems, Mansfield, Massachusetts, USA) with instrument compensation set in each experiment using single and/or two-color stained isotype control samples.
Pathologic examination of tissues.
Mice were sacrificed, and specimens of liver, skin, and intestine were taken for histopathologic analysis. All samples were placed in 10% neutral buffered formalin (Sigma Aldrich), embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histopathologic assessment of acute GVHD (2, 22).
Immunohistochemical and fluorescent staining of tissues.
For immunofluorescence detection of CD11c antigen, recipient spleens were frozen in O.C.T. embedding medium (Sakura Finetek USA Inc., Torrance, California, USA). Six-micrometer frozen sections were cut, air-dried, fixed in acetone, and rehydrated in PBS. Spleen sections were incubated with biotinylated hamster anti–mouse CD11c Ab (Pharmingen) and revealed with PE-conjugated streptavidin (Vector Laboratories, Burlingame, California, USA), respectively. Nonspecific binding of biotin and avidin was blocked by addition of excess avidin and biotin using the Blocking Kit from Vector Laboratories. Isotype-matched rat IgG (Pharmingen) was used as a negative control. After staining, the slides were coverslipped using antifade mounting medium (Vectashield, H-1200; Vector Laboratories) and observed under a fluorescent microscope (Nikon Microphot-FXA; Nikon Inc., Melville, New York, USA).
Immunohistochemical staining of spleen, liver, and skin was performed as previously described (15, 17). The sections of liver and skin were stained with rat anti-CD8 Ab (Pharmingen) followed by horseradish peroxidase–conjugated (HRP-conjugated) goat anti–rat IgG(Fab′)2 (Vector Laboratories) and were developed with a diaminobenzidine (DAB) substrate kit (Vector Laboratories). The spleen sections were stained with biotinylated hamster anti–mouse CD11c Ab followed by HRP-conjugated streptavidin and developed with DAB substrate (brown). After inactivating HRP using 3% H2O2-PBS, these spleen sections were stained with rat anti–mouse CD4 Ab followed by staining with HRP-conjugated goat anti–rat IgG(Fab′)2. The sections were developed with DAB-Ni substrate (gray-black) (Vector Laboratories). Isotype-matched rat IgG (Pharmingen) was used as a negative control.
Statistical analysis.
Survival data were analyzed by life table methods using the Mantel-Peto-Cox summary of χ2. P values smaller than 0.05 were considered significant.
Results
C3H.SW mouse–derived donor CD8+ T cells mediate GVHD in B6 recipients.
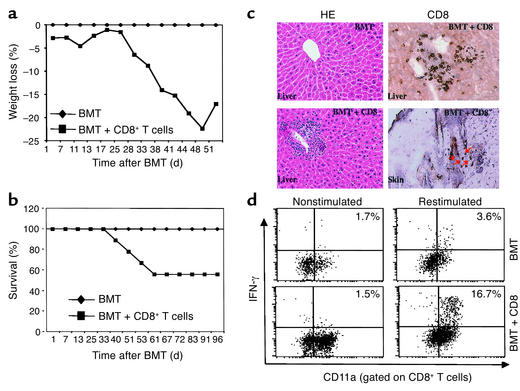
In previous studies (12), donor CD8+ T cells used for the induction of acute GVHD contained CD8+CD11c+ lymphoid DCs that could partially cross-present host antigens to activate CD8+ T cells (10, 23, 24). To investigate whether donor CD8+ T cells depleted of CD8+CD11c+ lymphoid DCs can induce lethal GVHD, we purified donor CD8+ T cells from C3H.SW mice by sequentially depleting CD11c+, CD11b+, CD4+, B220+, and NK1.1+ cells using magnetic cell sorting. Lethally irradiated B6 mice that received donor CD8+ T cells plus T–BM developed significant acute GVHD, as manifested by weight loss (Figure 1a), cutaneous inflammation (12 of 17 recipients), periportal infiltration of inflammatory cells, accumulation of CD8+ T cells in the liver and skin (Figure 1c), and death (7 of 17 recipients) (Figure 1b). Intracellular cytokine staining demonstrated that donor CD8+ T cells isolated from the recipient livers at 28 days after BMT were activated and secreted increased levels of IFN-γ (Figure 1d). In contrast, recipients infused with donor T–BM alone did not show any sign of GVHD (Figure 1). Thus, C3H.SW mouse–derived CD8+ T cells from which donor CD8+CD11c+ DCs are depleted can induce acute GVHD in irradiated B6 recipients by responding to the residual host APCs.
Figure 1.
Development of acute GVHD in B6 recipients transplanted with C3H.SW T–BM cells and CD8+ T cells. (a) Recipient weight loss and (b) actuarial survival rate. Recipients were conditioned with 9.5 Gy TBI and then administered 7 × 106 C3H.SW T–BM cells alone (n = 12; diamonds) or along with 3 × 106 C3H.SW CD8+ T cells (n = 17; squares). Data are derived from three separate experiments. (c) Infiltration of lymphocytes and CD8+ T cells in liver and skin (arrows) during GVHD. Livers and skin were harvested from mice and sectioned for histologic staining with hematoxylin and eosin (HE) and immunohistochemical staining with anti-CD8 Ab as described in Methods. Magnification: left, ×100; right, ×200. (d) Intracellular IFN-γ expression in hepatic CD8+ T lymphocytes. Hepatic lymphocytes were isolated from mice and restimulated in vitro in the presence or absence of anti-CD3 Ab plus MC57SV cells (H-2Db) irradiated with 30 Gy for 16 hours. Lymphocytes were then labeled with anti-CD8 and anti-CD11a Ab’s, fixed, permeabilized, and labeled with anti–IFN-γ Ab. The expression of intracellular IFN-γ in CD8+ T cells was quantitated by flow cytometry. Dot plots shown are from IFN-γ and CD11a labeling of gated CD8+ T cells.
Irradiation of B6 recipients induces the activation, maturation, and rapid elimination of host DCs.
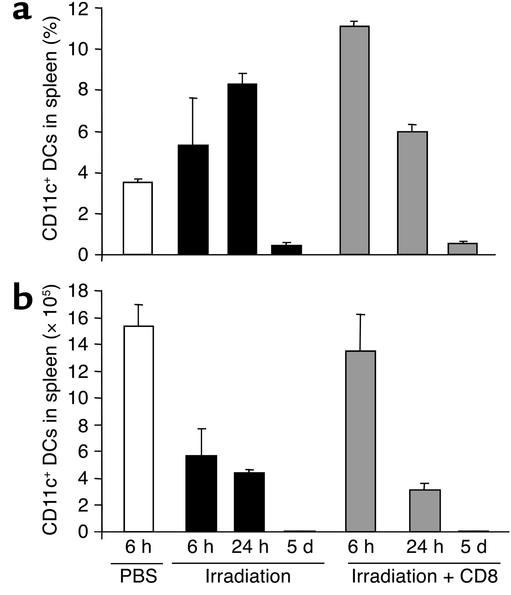
Since DCs are the APCs specialized for eliciting primary T cell responses (10), determining the fate of host DCs following TBI may illuminate how host APCs initiate donor CD8+ T cell–mediated GVHD. We first examined the fate of host DCs in the spleens of B6 mice following lethal TBI. Flow cytometry analysis showed that 6–24 hours after TBI, the density of host CD11c+ DCs increased substantially in recipient spleens (Figure 2a). However, TBI reduced the overall cellularity of recipient spleens by 73.7% and 87.4% by 6 hours and 24 hours, respectively. The absolute number of host CD11c+ DCs thus declined significantly by 6 hours following TBI, and by day 5 more than 99.0% of host DCs in the spleen were eliminated (Figure 2b). Infusion of donor CD8+ T cells immediately after TBI further increased the percentage of splenic CD11c+ DCs in TBI recipients at 6 and 24 hours, but did not rescue the overall loss of host CD11c+ DCs during this 5-day period (Figure 2, a and b). We likewise observed that DCs were diminished in the peripheral lymph nodes, blood, liver, and skin of recipients at 24 hours and were undetectable in these tissues by 5 days after irradiation (data not shown). The rapid elimination of host DCs during the first few days following TBI was surprising, since acute GVHD was not clinically and pathologically detected in the recipients until 1–2 weeks after allo-BMT (Figure 1).
Figure 2.
Elimination of CD11c+ DCs in the spleens of irradiated mice. Spleen cells were isolated from lethally irradiated B6 mice 6 hours, 24 hours, and 5 days after TBI with or without transplantation of C3H.SW CD8+ T cells, and CD11c+ DCs were enumerated by flow cytometry. (a) Percentage and (b) absolute number of CD11c+ DCs in the spleens of B6 mice injected with PBS, irradiated B6 mice injected with PBS, or irradiated B6 mice infused with C3H.SW CD8+ T cells. Data are representative of three experiments.
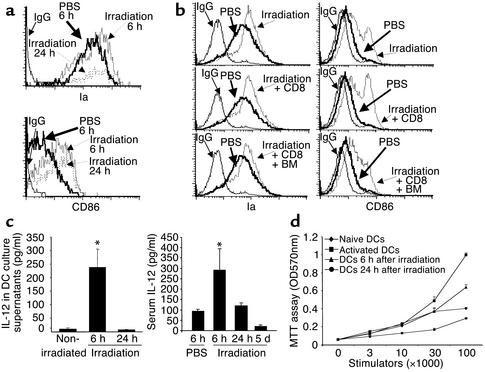
It is believed that activation of naive T cells depends on the maturation state of DCs in vivo (10). Several experiments were performed to examine whether host DCs might be functionally activated following irradiation but prior to their disappearance. As shown in Figure 3a, splenic DCs from irradiated mice expressed increased numbers of Ia and CD86 as early as 6 hours after irradiation; this increase was not related to whether or not T–BM and/or T cells were infused (Figure 3b). The increased expression of CD86 persisted as long as 24 hours, whereas Ia was downregulated at 24 hours (Figure 3a). When splenic DCs were isolated and cultured ex vivo for an additional 48 hours, DCs isolated from irradiated B6 mice at 6 hours, but not those isolated at 24 hours following TBI, produced significantly higher levels of IL-12 than the cells from nonirradiated mice did, consistent with the increased levels of serum IL-12 detected in these irradiated mice (Figure 3c). Moreover, DCs isolated from irradiated B6 mice at 6 hours following TBI showed greater capacity than did the cells from nonirradiated naive B6 mice to stimulate the allogeneic mixed lymphocyte reaction (Figure 3d). Taken together, these results indicate that the BMT-preparative irradiation rapidly induced the functional activation of host DCs prior to their disappearance.
Figure 3.
Irradiation upregulates the expression of antigen-presenting and costimulatory molecules on DCs. Splenic DCs were isolated from (a) irradiated mice at 6 hours and 24 hours after irradiation or (b) irradiated mice infused with donor CD8+ T cells or donor CD8+ T cells + T–BM cells. These purified DCs were double stained with anti-CD11c Ab coupled with anti-Ia Ab or anti-CD86 Ab as described in Methods. The expression of Ia and CD86 antigens on the CD11c+ cell population was analyzed by flow cytometry. Isotype-matched IgG was used as control. (c) IL-12 in serum from nonirradiated and irradiated B6 mice at 6 hours, 24 hours, and 5 days following TBI or in the supernatants of cultured host DCs was examined by ELISA. Host splenic DCs were separately purified from the spleen of B6 mice at 6 and 24 hours following TBI or from nonirradiated mice as described in Methods. The supernatants were collected from the cultures of DCs at 48 hours. *P < 0.05 compared with samples from nonirradiated mice or irradiated mice at 24 hours and 5 days after TBI. (d) Host DCs were isolated from nonirradiated B6 mice (diamonds) and from B6 mice at 6 hours (triangles) and 24 hours (circles) after irradiation. These cells were cocultured with BALB/c mouse–derived CD4+ T cells to examine their capacity to stimulate an allogeneic mixed lymphocyte reaction. Activated DCs that were induced by culturing naive B6 DCs in the presence of GM-CSF + TNF-α for 48 hours were used as positive control (squares). One representative experiment of three is shown.
Host DCs intimately interact with infused donor naive CD8+ T cells in vivo.
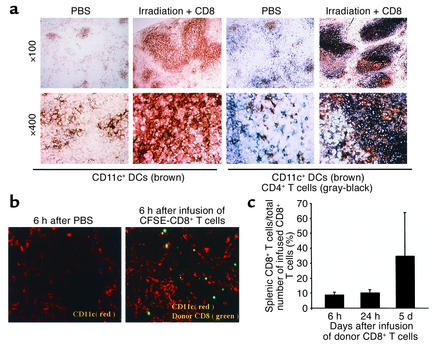
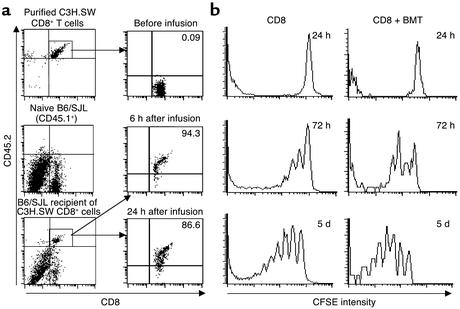
Direct contact between host APCs and resting donor T cells is essential for initiating primary immune responses (10, 25). Therefore, if host DCs are essential to allogeneic donor T cell activation, direct proximity of DCs to T cells should be detectable before the disappearance of host DCs following irradiation. To test this hypothesis, C3H.SW CD8+ T cells were labeled with the fluorescent dye CFSE and transplanted into lethally irradiated B6 recipients. Two-color immunohistochemical staining of spleen sections with anti-CD11c and anti-CD4 Ab’s revealed that CD11c+ DCs were significantly concentrated in the T cell areas of the spleens from B6 recipients at 6 hours following TBI and cell transplantation (Figure 4a). Fluorescence microscopy showed that donor CFSE-CD8+ T cells were readily detected in the spleens by 6 hours following infusion. These CFSE-CD8+ T cells were seen in direct proximity to host DCs in situ (Figure 4b). Flow cytometry analysis showed that about 5–10% of all infused donor CFSE-CD8+ T cells were recruited into the spleen by 6–24 hours following transplantation, and this number increased five- to tenfold by day 5 (Figure 4c). In contrast, CFSE-CD8+ T cells were not detected in the skin and were only sparsely detected in the liver (less than 0.3% of all hepatic leukocytes) at 24 hours after infusion (data not shown). Thus, transplanted donor CD8+ T cells form an intimate interaction with preterminal host DCs in the T cell areas of the spleen where primary T cell response is initiated.
Figure 4.
Donor CD8+ T cells are rapidly recruited to the T cell areas of recipient spleens, in direct proximity to host CD11c+ DCs. CFSE-labeled CD8+ T cells (2 × 106) derived from C3H.SW mice (CD45.2+) were intravenously injected into lethally irradiated B6/SJL mice (CD45.1+). (a) Aggregation of CD11c+ DCs in the T cell areas of the spleens of irradiated mice. Cryosections of spleen harvested from lethally irradiated B6 mice 6 hours after TBI were immunostained with anti-CD11c Ab (brown) and anti-CD4 Ab (gray-black). Magnification: top row, ×100; bottom row, ×400. Data are representative of three experiments. (b) Spleens were taken from B6 mice 6 hours after PBS injection and from irradiated B6 mice 6 hours after infusion of CFSE-labeled C3H.SW CD8+ T cells (green) and cryosectioned for immunofluorescent staining with anti-CD11c Ab (red). Magnification, ×100. (c) Splenocytes were prepared and counted at the indicated timepoints. Cells were sequentially labeled with Cychrome-conjugated anti-CD8 Ab coupled with biotinylated anti-CD45.2 and streptavidin-conjugated PE. The percentage of CD8+CD45.2+ cells was obtained by flow cytometry analysis. The recruitment rate (percentage) of donor CD8+ T cells in spleen was calculated by dividing the absolute number of donor CD8+ T cells recovered from the spleens of irradiated recipient mice by the total number of intravenously injected donor CD8+ T cells.
Allogeneic donor T cells rapidly upregulate activation antigens and proliferate shortly after infusion.
We next assessed the early responsiveness of donor CD8+ T cells in the recipients following TBI. Six hours after transplantation of donor C3H.SW CD8+ T cells (CD45.2+) into lethally irradiated B6/SJL recipients (CD45.1+), flow cytometry analysis demonstrated increased expression of the activation antigen CD25 on about 90% of donor T cells recovered from recipient spleens (Figure 5a). Similarly, the expression of CD69 antigen on these donor CD8+ T cells was increased while that of CD62L antigen was decreased (data not shown), indicating rapid activation of allogeneic T cells in vivo (2). At 24 hours after transplantation, about 2.4% of transplanted CFSE-labeled donor C3H.SW CD8+ T cells had divided. Donor T cell proliferation amplified extensively over the next several days, such that as many as seven divisions per T cell were detected by day 5, whether or not donor T–BM cells were included in the infusion (Figure 5b). These results suggest that allogeneic CD8+ T cells are activated before the elimination of host DCs.
Figure 5.
Activation of donor naive CD8+ T cells upon intravenous injection into lethally irradiated B6 recipients. (a) Naive CD8+CD45.2+ C3H.SW T cells (2 × 106) were intravenously injected into lethally irradiated B6/SJL recipients (CD45.1+). At the indicated timepoints, splenocytes were prepared from these mice and tricolor-stained with anti-CD8, anti-CD45.2, and anti-CD25 Ab’s, which were separately revealed with FITC, Cychrome, and PE. After gating on CD45.2+ donor–derived T cells, the expression of CD25 on CD8+ T cells was analyzed by flow cytometry. (b) CFSE-labeled CD8+ T cells (2 × 106) from C3H.SW donor mice (CD45.2) were transferred with or without mixed donor T–BM cells into lethally irradiated B6/SJL recipient mice (CD45.1). At the indicated timepoints after transplantation, splenocytes were prepared from these mice, stained with anti-CD8 Ab conjugated with Cychrome, and then sequentially labeled with biotinylated anti-CD45.2 and streptavidin-conjugated PE. The division of donor CD8+ T cells in vivo was analyzed by flow cytometry by gating on CD8+CD45.2+ T cells. One representative experiment of three is shown.
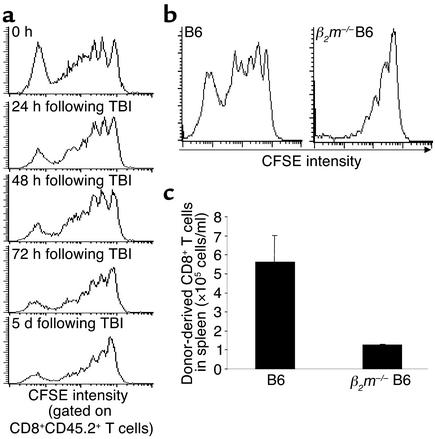
To further examine the relationship between proliferation of donor CD8+ T cells and the disappearance of host DCs, C3H.SW CFSE-CD8+ T cells were transplanted into B6 recipients at 0, 24, 48, 72, and 120 hours following TBI. Five days after the cell transplantation, activation and expansion of donor CD8+ T cells were analyzed by flow cytometry. Compared with the transplantation of donor T cells immediately after TBI, delayed infusion of these donor CFSE-CD8+ T cells into B6 recipients at 24–120 hours after TBI significantly inhibited their division and proliferation in the recipients (Figure 6a). The absolute number of donor CD8+ T cells that underwent more than seven divisions in recipient spleen were markedly decreased from 3.0 × 105 ± 0.4 × 105 at 0 hours to 1.06 × 105 ± 0.5 × 105 at 24 hours, 1.43 × 105 ± 0.33 × 105 at 48 hours, 0.57 × 105 ± 0.1 × 105 at 72 hours, and 0.68 × 105 ± 0.04 × 105 at 120 hours after TBI. Identically, when β2m–/– B6 mice were substituted for B6 recipient mice, donor CD8+ T cell division was remarkably inhibited, largely limited to one or two divisions by day 5 (Figure 6b, lower panel). It is not clear whether this limited allogeneic T cell proliferation in β2m–/– B6 recipients might be induced by recognition of free MHC class I chains by donor CD8+ T cells (26) or whether memory CD8+ T cells retain their functional characteristics and proliferate in the absence of MHC class I (27). Nevertheless, this reduction in cell division resulted in a markedly reduced (fivefold) expansion of donor CD8+ T cells in β2m–/– B6 mice by day 5 after transplantation (Figure 6c). Thus, activation and proliferation of donor CD8+ T cells are closely associated with the disappearance of host DCs in vivo, which requires the presence of functional MHC class I molecules on the host APCs.
Figure 6.
The activation and proliferation of allogeneic CD8+ T cells depend on the presence of host DCs and require MHC class I expression on host cells. (a) CFSE-labeled CD8+ T cells (2 × 106) from C3H.SW mice (CD45.2+) were transplanted into irradiated B6 recipients (CD45.1+) at the indicated timepoints after TBI. Five days later, splenocytes were prepared from these mice for flow cytometry analysis as described in Figure 5b legend. (b) C3H.SW CFSE-CD8+CD45.2+ T cells (2 × 106) were intravenously transferred into lethally irradiated β2m–/– mice or congenic B6/SJL (CD45.1+) recipients. Splenocytes were prepared from these mice, and the division of donor CD8+ T cells was analyzed by flow cytometry. The absolute number (c) of C3H.SW CD8+ T cells in the spleens of recipients 5 days after transplantation was calculated based on the flow cytometry analysis. Data shown derive from five independent experiments. One representative experiment of three is shown.
Early priming of donor CD8+ T cells by host APCs is sufficient to trigger allogeneic T cells to mediate lethal GVHD in β2m–/– → B6 chimeric mice.
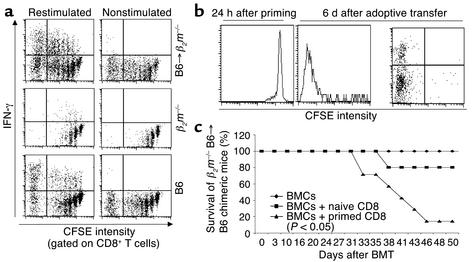
Since host DCs were eliminated in the spleens of mice during the first few days after irradiation in parallel with donor T cell proliferation, we asked whether the early exposure of donor T cells to activated, preterminal DCs was sufficient to trigger acute GVHD. To test this hypothesis, CFSE-labeled C3H.SW (CD45.2+) CD8+ T cells were infused into lethally irradiated B6/SJL recipients (CD45.1+). Twenty-four to 36 hours later, recipient mice were sacrificed and C3H.SW CD8+ T cells were isolated from recipient spleens and lymph nodes by positive selection based on the expression of CD45.2 antigen. The purity of separated CD8+CD45.2+ T cells was more than 96%, and contamination of CD11c+ cells was undetectable by flow cytometry. These in vivo primed CD8+ T cells were then adoptively transferred into lethally irradiated β2m–/– B6 recipients, and the proliferation and differentiation of these T cells were examined 6 days after adoptive transfer. As shown in Figure 7a, these in vivo primed C3H.SW CD8+ T cells spontaneously underwent at least seven divisions and secreted high levels of IFN-γ in the secondary β2m–/– B6 recipient mice. In contrast, unprimed C3H.SW CD8+ T cells were unable to extensively divide and differentiate into IFN-γ–producing cells in β2m–/– recipients (Figure 7a).
Figure 7.
Brief priming by host DCs triggers the committed differentiation of donor naive CD8+ T cells into effectors. (a) Naive C3H.SW CFSE-CD8+ T cells were intravenously injected into lethally irradiated B6/SJL (CD45.1+) mice. After 24 to 36 hours, these primed C3H.SW CFSE-CD8+ T cells were then purified and adoptively transferred into secondary irradiated β2m–/– recipient mice. Six days later, cells from the spleens and lymph nodes of secondary recipients were cultured in the presence or absence of CD3 Ab plus MC57SV cells irradiated with 30 Gy for measuring IFN-γ production as described in Methods. Dot plots shown represent IFN-γ and CFSE intensity measured in gated CD8+ T cells. Naive unprimed C3H.SW CFSE-CD8+ T cells were injected into lethally irradiated B6/SJL mice (CD45.1+) and β2m–/– mice as control. (b) C3H.SW CFSE-CD8+ T cells were stimulated ex vivo by host CD11c+ DCs for 24 hours, recovered, sorted, and adoptively transferred into irradiated β2m–/– mice. Six days later, cells from spleens and lymph nodes were separated from the secondary recipients and stained with anti-CD8 Ab for measuring donor CD8+ T cell divisions. These cells were also cultured for 16 hours as described in a, to measure IFN-γ secretion. (c) Survival rate of β2m–/– B6 → B6 BM chimeric mice that received either C3H.SW T–BM alone, C3H.SW T–BM + C3H.SW CD8+ T cells, or C3H.SW T–BM + C3H.SW CD8+ T cells that were primed ex vivo by B6 CD11c+ DCs. Representative results from two independent experiments are shown.
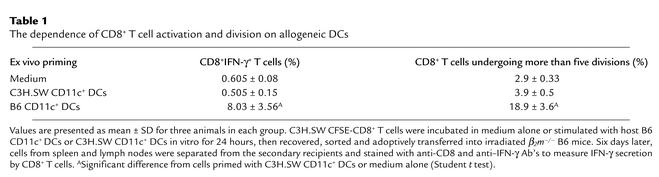
To further investigate whether short-lived but activated host CD11c+ DCs might be responsible for priming donor T cells in vivo, CFSE-labeled C3H.SW CD8+ T cells (CD45.2+) were cultured ex vivo with CD11c+ DCs that were isolated from spleens and lymph nodes of B6/SJL (CD45.1+) mice 6 hours after lethal irradiation. Twenty-four hours later, these primed C3H.SW CD8+ T cells were magnetically sorted from the cultures to completely remove host DCs and adoptively transferred into the irradiated β2m–/– B6 recipients and β2m–/– → B6 chimeric mice. As shown in Figure 7b and Table 1, these C3H.SW CD8+ T cells primed ex vivo by host CD11c+ DCs underwent as many as seven divisions and differentiated into effectors secreting high levels of IFN-γ following adoptive transfer into β2m–/– mice. In contrast, priming of C3H.SW CD8+ T cells with syngeneic C3H.SW CD11c+ DCs or medium alone failed to induce the proliferation and functional differentiation of these T cells into effectors secreting IFN-γ under the same conditions. (Table 1). Finally, six of seven β2m–/– B6 → B6 BM chimeric recipients that received C3H.SW T–BM plus CD8+ T cells that were primed ex vivo by host CD11c+ DCs developed severe GVHD by day 30 and died from lethal GVHD (Figure 7c). In contrast, only one of five β2m–/– → B6 BM chimeric recipients transplanted with unprimed donor CD8+ T cells showed any signs of GVHD, with a delayed onset (Figure 7c; P < 0.05 compared with those recipients accepting host DC–primed donor CD8+ T cells), consistent with previous observations (12). Thus, exposure of donor CD8+ T cells to host DCs for 24 hours was sufficient to trigger the proliferation and differentiation of CD8+ T cells and could induce acute GVHD.
Table 1.
The dependence of CD8+ T cell activation and division on allogeneic DCs
Discussion
The studies in this model of MHC-matched, miHA-mismatched allo-BMT demonstrate that preparative TBI for allo-BMT can rapidly induce the functional maturation of host DCs, which trigger the committed differentiation of allogeneic CD8+ T cells. DCs are sentinel APCs distributed throughout tissues as an immature form (10). In response to various stimuli (including infectious agents, inflammatory cytokines, and endogenous signals released by cells undergoing stress, damage, and necrosis), immature DCs can be activated in vivo (10, 15, 28, 29). We found that preparative irradiation resulted in significantly increased serum levels of IL-12 and functional activation of host DCs within as little as 6 hours. Since we found that freshly isolated splenic DCs underwent spontaneous maturation upon in vitro culture (data not shown), consistent with others’ observations (16, 28), it was difficult to evaluate whether ex vivo irradiation might directly affect the maturation of freshly isolated host DCs. Previous studies indicate that preparative irradiation for BMT results in the damage of host tissues and release of inflammatory cytokines such as IL-1 and TNF-α (30–35), which are closely related to the increased risk of lethal GVHD in animal models as well as in human BMT (1, 30, 33, 36). Accordingly, the early released inflammatory cytokines and damaged tissues undergoing stress and necrotic death may play a critical role in inducing the rapid activation of host DCs in lethally irradiated recipients. Thus, preventing the activation of host DCs induced by an intensive myeloablative regimen and other risk factors, particularly in the first few days following clinical BMT, might be helpful for reducing the incidence of acute GVHD.
We found that TBI significantly diminished the number of host splenic DCs in the recipients soon after irradiation and eliminated more than 99% of them by day 5. Such elimination of host DCs was not related to the redistribution of host DCs in vivo, since there were not signs of DC accumulation in other tissues of irradiated recipients. Several previous studies have demonstrated that systemic inflammation can induce the disappearance of activated DCs within 24 to 48 hours in the T cell areas of spleen (10, 15, 37). After terminal maturation and interaction with lymphocytes, DCs die in situ in the secondary lymphoid tissues by apoptosis (10, 29, 37). Thus, preparative irradiation, through its simultaneous induction of DC maturation and its direct toxicity to DC precursor cells, results in the elimination of host DCs in the recipients.
Accumulating evidence points to the fact that the early interaction between allogeneic T cells and residual host APCs immediately after allo-BMT is critical for eliciting acute GVHD (12, 38–42). Compared with the transplantation of donor lymphocytes on the day of BMT, delayed lymphocyte infusion of allogeneic T cells into the recipient mice between day 7 and day 21 after BMT results in decreased generation of cytolytic and helper T cells (42) and a reduced incidence of lethal GVHD (38–42). Although it has been suggested that the presence of fewer host APCs in the recipients after allo-BMT might account for decreased severity of GVHD in these delayed lymphocyte infusion models (40), the kinetic window in which host APCs function to elicit acute GVHD has been uncertain. We observed that the elimination of host DCs by TBI did not prevent the activation and proliferation of allogeneic CD8+ T cells that were transplanted into the recipients immediately after irradiation. In contrast, delayed infusion of donor CD8+ T cells into the B6 recipients between 3 days and 5 days after TBI substantially inhibited the activation and rapid proliferation of these allogeneic T cells in vivo. We conclude that activated host DCs trigger the activation and proliferation of allogeneic CD8+ T cells prior to their decay and disappearance. Delayed infusion of donor T cells may result in a significant reduction in the chance for donor T cells to encounter activated host DCs in the recipients, thereby blunting the effect of host DCs on triggering the activation and functional differentiation of host-specific donor CD8+ T cells.
Our experiments address the issue of the duration of interaction between host DCs and donor T cells required to trigger allogeneic CD8+ T cells to differentiate into GVHD effectors. When C3H.SW CD8+ T cells were primed ex vivo for 24 hours by activated B6 CD11c+ DCs and adoptively transferred into β2m–/– → B6 chimeric mice, the sensitized allogeneic CD8+ T cells could lead to the lethal GVHD in these chimeric recipients by 30 days. These results indicate that host DCs can prime donor CD8+ T cells within 24 hours. Using engineered fibroblast APCs expressing B7.1 and pSigOVA(257–264), van Stipdonk et al. recently showed that priming naive OT-I CD8+ T cells for 2 hours in an in vitro culture was sufficient for inducing rapid division and CTL activity of these OT-I T cells in the absence of any further antigenic stimulation (43). Two other in vivo studies based on animal models showed that either bacterial or malaria antigen–specific transgenic naive CD8+ T cells can become committed to differentiation into protective effectors secreting high levels of IFN-γ after initial antigen encounter in vivo for 24 hours (44). Our present results support these conclusions and extend this to cellular allogeneic miHAs in an in vivo murine GVHD model analogous to human clinical BMT. Thus, antigen stimulation is re-quired only briefly immediately after BMT for priming donor T cells to trigger their proliferation and committed differentiation into functional effectors of GVHD.
Previous studies have demonstrated that both professional and nonprofessional APCs may support alloresponses (10, 11, 13, 45). Although the present study establishes the critical effect of host DCs at a very early stage following allo-BMTon initiating allogeneic CD8+ T cell–mediated acute GVHD in vivo, we do not rule out the role of other host APCs such as macrophages, B cells, and some semiprofessional APCs in vivo in presenting miHAs to activate donor CD8+ T cells. Moreover, we have previously observed the presence of a small number of radio-resistant host APCs in recipients 4 months after allo-BMT. Thus, it is possible that maturation of primed CD8+ T cells into GVHD effectors following adoptive transfer into β2m–/– → B6 chimeric mice could have been aided by the few radio-resistant host APCs. This raises the interesting possibility that such residual host APCs could be important for amplifying GVHD response during the effector phase, perhaps in the host target tissues. Further studies are necessary for dissecting the role of distinct APCs in regulating these early versus later events in the induction of acute GVHD.
The findings of this study further extend our previous observation at a cellular mechanism level that host APCs are essential for allogeneic CD8 T cells to induce acute GVHD in an miHA-mismatched mouse model (12). After rapid priming for as little as 24 hours by host DCs, allogeneic CD8+ T cells became committed to differentiation into functional effectors mediating acute GVHD. This suggests that a blockade of DC–T cell interaction should be required for only a short period after BMT, until host DCs have disappeared. Despite the phenotypic manifestation of GVHD in the second week after BMT and beyond, it is the first few days after BMT in which GVHD is initiated. It is this period that should be targeted in order to ameliorate, and hopefully prevent, the subsequent development of GVHD.
Acknowledgments
We greatly appreciate the technical support given by Diane Giannola and Gerard Joe (Division of Hematology and Oncology, Department of Medicine, University of Pennsylvania School of Medicine). This work was supported by grants from the Leukemia and Lymphoma Society of America.
References
- 1.Ferrara JL, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant. 1999;5:347–356. doi: 10.1016/s1083-8791(99)70011-x. [DOI] [PubMed] [Google Scholar]
- 2.Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs.-host disease: pathobiology and management. Exp Hematol. 2001;29:259–277. doi: 10.1016/s0301-472x(00)00677-9. [DOI] [PubMed] [Google Scholar]
- 3.den Haan JM, et al. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science. 1998;279:1054–1057. doi: 10.1126/science.279.5353.1054. [DOI] [PubMed] [Google Scholar]
- 4.den Haan JM, et al. Identification of a graft versus host disease-associated human minor histocompatibility antigen. Science. 1995;268:1476–1480. doi: 10.1126/science.7539551. [DOI] [PubMed] [Google Scholar]
- 5.Rotzschke O, Falk K, Wallny HJ, Faath S, Rammensee HG. Characterization of naturally occurring minor histocompatibility peptides including H-4 and H-Y. Science. 1990;249:283–287. doi: 10.1126/science.1695760. [DOI] [PubMed] [Google Scholar]
- 6.Goulmy E. Minor histocompatibility antigens: from T cell recognition to peptide identification. Hum Immunol. 1997;54:8–14. doi: 10.1016/s0198-8859(97)00007-4. [DOI] [PubMed] [Google Scholar]
- 7.Blazar BR, Korngold R, Vallera DA. Recent advances in graft-versus-host disease (GVHD) prevention. Immunol Rev. 1997;157:79–109. doi: 10.1111/j.1600-065x.1997.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 8.Korngold R, Sprent J. Variable capacity of L3T4+ T cells to cause lethal graft-versus-host disease across minor histocompatibility barriers in mice. J Exp Med. 1987;165:1552–1564. doi: 10.1084/jem.165.6.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sprent J, Schaefer M, Lo D, Korngold R. Functions of purified L3T4+ and Lyt-2+ cells in vitro and in vivo. Immunol Rev. 1986;91:195–218. doi: 10.1111/j.1600-065x.1986.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 11.Lechler R, Ng WF, Steinman RM. Dendritic cells in transplantation — friend or foe? Immunity. 2001;14:357–368. doi: 10.1016/s1074-7613(01)00116-9. [DOI] [PubMed] [Google Scholar]
- 12.Shlomchik WD, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 13.Kosaka H, Surh CD, Sprent J. Stimulation of mature unprimed CD8+ T cells by semiprofessional antigen-presenting cells in vivo. J Exp Med. 1992;176:1291–1302. doi: 10.1084/jem.176.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao EK, Kosaka H, Surh CD, Sprent J. T cell contact with Ia antigens on nonhemopoietic cells in vivo can lead to immunity rather than tolerance. J Exp Med. 1991;174:435–446. doi: 10.1084/jem.174.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 16.Vremec D, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoneyama H, et al. Pivotal role of TARC, a CC chemokine, in bacteria-induced fulminant hepatic failure in mice. J Clin Invest. 1998;102:1933–1941. doi: 10.1172/JCI4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson ML, Farmer ER. Graft-versus-host reactions in dermatology. J Am Acad Dermatol. 1998;38:369–396. doi: 10.1016/s0190-9622(98)70495-5. [DOI] [PubMed] [Google Scholar]
- 19.Murali-Krishna K, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, et al. Induction of dendritic cell differentiation by granulocyte-macrophage colony-stimulating factor, stem cell factor, and tumor necrosis factor alpha in vitro from lineage phenotypes-negative c-kit+ murine hematopoietic progenitor cells. Blood. 1997;90:4842–4853. [PubMed] [Google Scholar]
- 21.Zhang Y, et al. Bifurcated dendritic cell differentiation in vitro from murine lineage phenotype-negative c-kit+ bone marrow hematopoietic progenitor cells. Blood. 1998;92:118–128. [PubMed] [Google Scholar]
- 22.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 23.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferlazzo G, et al. Dendritic cells efficiently cross-prime HLA class I-restricted cytolytic T lymphocytes when pulsed with both apoptotic and necrotic cells but not with soluble cell-derived lysates. Int Immunol. 2000;12:1741–1747. doi: 10.1093/intimm/12.12.1741. [DOI] [PubMed] [Google Scholar]
- 25.Geijtenbeek TB, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 26.Glas R, et al. Major histocompatibility complex class I-specific and -restricted killing of beta 2-microglobulin-deficient cells by CD8+ cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1992;89:11381–11385. doi: 10.1073/pnas.89.23.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murali-Krishna K, et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 28.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 29.Sauter B, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrara JL. The cytokine modulation of acute graft-versus-host disease. Bone Marrow Transplant. 1998;21(Suppl. 3):S13–S15. [PubMed] [Google Scholar]
- 31.Ferrara JL. Pathogenesis of acute graft-versus-host disease: cytokines and cellular effectors. J Hematother Stem Cell Res. 2000;9:299–306. doi: 10.1089/15258160050079407. [DOI] [PubMed] [Google Scholar]
- 32.Hill GR, Cooke KR, Brinson YS, Bungard D, Ferrara JL. Pretransplant chemotherapy reduces inflammatory cytokine production and acute graft-versus-host disease after allogeneic bone marrow transplantation. Transplantation. 1999;67:1478–1480. doi: 10.1097/00007890-199906150-00015. [DOI] [PubMed] [Google Scholar]
- 33.Hill GR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 34.Hill GR, Krenger W, Ferrara JL. The role of cytokines in acute graft-versus-host disease. Cytokines Cell Mol Ther. 1997;3:257–266. [PubMed] [Google Scholar]
- 35.Hill GR, et al. Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J Clin Invest. 1999;104:459–467. doi: 10.1172/JCI6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–2759. [PubMed] [Google Scholar]
- 37.Sousa CR, et al. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blazar BR, et al. Host T cells resist graft-versus-host disease mediated by donor leukocyte infusions. J Immunol. 2000;165:4901–4909. doi: 10.4049/jimmunol.165.9.4901. [DOI] [PubMed] [Google Scholar]
- 39.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3192–3204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 40.Johnson BD, Becker EE, LaBelle JL, Truitt RL. Role of immunoregulatory donor T cells in suppression of graft-versus-host disease following donor leukocyte infusion therapy. J Immunol. 1999;163:6479–6487. [PubMed] [Google Scholar]
- 41.Johnson BD, Becker EE, Truitt RL. Graft-vs.-host and graft-vs.-leukemia reactions after delayed infusions of donor T-subsets. Biol Blood Marrow Transplant. 1999;5:123–132. doi: 10.1053/bbmt.1999.v5.pm10392958. [DOI] [PubMed] [Google Scholar]
- 42.Johnson BD, Truitt RL. Delayed infusion of immunocompetent donor cells after bone marrow transplantation breaks graft-host tolerance allows for persistent antileukemic reactivity without severe graft-versus-host disease. Blood. 1995;85:3302–3312. [PubMed] [Google Scholar]
- 43.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 44.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korngold R, Leighton C, Mobraaten LE, Berger MA. Inter-strain graft-vs.-host disease T-cell responses to immunodominant minor histocompatibility antigens. Biol Blood Marrow Transplant. 1997;3:57–64. [PubMed] [Google Scholar]