Abstract
Growth hormone secretagogues (GHSs) stimulate GH secretion and food intake. GHS receptor (GHS-R) mRNA has been identified mainly in the arcuate nucleus (Arc) and ventromedial nucleus of the hypothalamus and in the pituitary. Ghrelin, an endogenous ligand for GHS-R, has recently been purified from rat stomach. Although ghrelin is also expressed in the hypothalamus, the physiological significance of the ghrelin/GHS-R system is still unknown. We have created transgenic (Tg) rats expressing an antisense GHS-R mRNA under the control of the promoter for tyrosine hydroxylase (TH), thus selectively attenuating GHS-R protein expression in the Arc. Tg rats had lower body weight and less adipose tissue than did control rats. Daily food intake was reduced, and the stimulatory effect of GHS treatment on feeding was abolished in Tg rats. GH secretion and plasma insulin-like growth factor-I levels were reduced in female Tg rats. These results suggest that GHS-R in the Arc is involved in the regulation of GH secretion, food intake, and adiposity.
Introduction
Growth hormone secretagogues (GHSs), which were developed from met-enkephalin based on conformational energy calculations, peptide chemistry, and biological activity, stimulate GH secretion via a specific receptor (1). An intracerebroventricular (ICV) injection of GHS also stimulates food intake in freely feeding rats (2). The GHS receptor (GHS-R) was cloned and found to be a member of the G protein–coupled receptor superfamily (3). The expression of GHS-R mRNA is observed by in situ hybridization or an RNase protection assay mainly in the arcuate nucleus (Arc) and ventromedial nucleus of the hypothalamus and in the pituitary (4). Ghrelin, an endogenous ligand for GHS-R, has recently been isolated from stomach extracts of rats and subsequently cloned in rats and humans (5). Ghrelin-producing cells are found in the hypothalamus as well as in the stomach (5). As expected, ICV administration of ghrelin stimulated GH secretion and food intake in rats (6, 7). Daily peripheral administration of ghrelin caused weight gain by reducing fat utilization in mice and rats (8). However, the physiological role of endogenous ghrelin in the hypothalamus is still unknown.
To attenuate GHS-R expression in vivo, we attempted to create transgenic (Tg) rats with impaired GHS-R function in the hypothalamus, especially in the Arc. For this purpose, we used a construct that expresses GHS-R–specific antisense RNA under the control of the promoter for tyrosine hydroxylase (TH). TH is the rate-limiting enzyme in catecholamine biosynthesis and is a marker for the dopaminergic neurons in the hypothalamus. TH-like immunoreactivity is present in most neurons in the ventral part of the Arc that contain GH-releasing hormone (GHRH) (9). GHS-R mRNA hybridizing cells show an extensive overlap with GHRH-expressing neurons (10). These results suggest that a certain number of GHS-R–expressing neurons in the Arc also contain TH. Tg mice bearing a fusion gene containing the TH promoter and the coding region of the human GH gene have been generated, and these Tg mice showed human GH–like immunoreactivity in all the catecholaminergic neurons in the hypothalamus (11). Based on this report, an antisense GHS-R mRNA under the control of the TH promoter would be expected to suppress GHS-R expression in the Arc. Therefore, in the present study we have generated Tg rats that express an antisense GHS-R mRNA under the control of the TH promoter to determine the physiological role of the ghrelin/GHS-R system in the hypothalamus.
Methods
Generation of Tg rats.
To construct the antisense GHS-R fusion gene, a synthetic 108-nucleotide DNA fragment spanning the 5′ extracellular region of GHS-R was cloned in the antisense orientation into the vector 4.5THpAL+ (kindly provided by Dona Chikaraishi), which contains 4.5 kb of the upstream region of the rat TH gene (11). The antisense orientation of the cloned fragments was verified by DNA sequencing. A 4.8-kb EcoRV-SalI fragment was then microinjected into fertilized eggs of slc:SD rats. Insertion of the DNA was confirmed by PCR analysis of tail DNA. In the experiments described below, the wild-type (slc:SD) inbred strain was used as the control group. All experiments were approved by the Animal Research Committee of Nippon Medical School.
RNA isolation and analysis.
Total RNA was extracted from tissues of Tg rats, and GHS-R antisense mRNA was detected using RT-PCR. cDNA was obtained from 1 μg of total RNA using avian myeloblastosis virus reverse transcriptase (Takara Biomedicals, Tokyo, Japan). The synthesized cDNA was amplified by PCR with sense primer 5′-CTGCAGGAATTCCAGCGGCA-3′ and antisense primer 5′-TTTAAGGGCACGGGCTGCAG-3′. PCR amplification involved an initial period of denaturation at 94°C for 3 minutes followed by 30 cycles consisting of 1 minute of denaturation at 94°C, 1 minute of annealing at 62°C, and 1 minute of extension at 72°C. The PCR products were separated on a 2% agarose gel, and the bands were visualized with ethidium bromide staining.
In situ hybridization.
To make an RNA probe for TH, a 475-bp fragment (nucleotides 789–1263) of the rat TH gene was amplified by RT-PCR, subcloned into pGEM-T Easy Vector (Promega Corp., Madison, Wisconsin, USA), and labeled with Biotin RNA Labeling Mix (Roche Diagnostics GmbH, Mannheim, Germany). For a GHS-R antisense probe, a synthetic 108-nucleotide DNA fragment of the GHS-R gene was also subcloned into pGEM-T Easy Vector and labeled using the DIG RNA Labeling Kit (Roche Diagnostics GmbH). Rats were perfused with paraformaldehyde, and brain was removed and kept in fixative containing 20% sucrose. Frozen 8-μm sections of brain were cut. Hybridization was performed at 42°C for 12 hours, and then slides were washed. Hybridized TH probes were detected using the TSA Biotin System (NEN Life Science Products Inc., Boston, Massachusetts, USA) and NBT/BCIP solution (Roche Diagnostics GmbH). Hybridized GHS-R antisense probes were also detected with the TMB substrate kit (Vector Laboratories, Burlingame, California, USA).
Western blot analysis.
Hypothalamic nuclei of Tg and control rats were isolated as described by Palkovits (12). Tissues were lysed in RIPA buffer (50 mM Tris-HCl at pH 8.0, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, 150 mM NaCl, and 0.5% deoxycholic acid) containing 1 mM phenylmethylsulfonyl fluoride (Sigma Aldrich, St. Louis, Missouri, USA), then homogenized with a glass homogenizer and centrifuged at 15,000 g for 15 minutes. The protein concentrations of the supernatants were determined with the DC protein assay (Bio-Rad Laboratories Inc., Hercules, California, USA). Equal amounts of each sample were separated by electrophoresis through 10% sodium dodecyl sulfate–polyacrylamide gels as described previously (13). Gels were then blotted onto nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA). The membranes were incubated overnight at 4°C with the antibody against GHS-R (1:1,000 dilution) (13). Immunocomplexes were visualized with the ECL Western blotting analysis system (Amersham Pharmacia Biotech). As an internal control, the levels of β-tubulin were also examined using the anti–rat β-tubulin monoclonal antibody (clone TUB 2.1; ICN Biomedicals Inc., Aurora, Ohio, USA).
Immunocytochemistry.
Rats were anesthetized with pentobarbital and perfused via an intracardiac cannula with PBS followed by 4% paraformaldehyde. The brain was removed, left overnight in 4% paraformaldehyde, and then transferred to 20% sucrose/PBS. Sections (30 μm) were cut with a sledge microtome and mounted onto gelatinized slides. Immunocytochemistry was performed using the antibody against GHS-R as previously described (13). Briefly, sections were incubated in the primary antiserum (1:100 dilution) for 48 hours at room temperature. Immunostaining was done using the avidin-biotin complex method (Vectastain ABC Elite kit; Vector Laboratories). The reaction product was visualized using a nickel-intensified 3,3′-diaminobenzidine reaction (Sigma Aldrich). Immunocytochemistry for TH was performed using an anti-TH polyclonal antibody (1:100,000 dilution; Chemicon International, Temecula, California, USA) and visualized using the VIP substrate kit (Vector Laboratories). For double-staining immunocytochemistry, 20-μm sections were processed for immunocytochemical detection of GHS-R using the VIP substrate kit (Vector Laboratories). Every third section was incubated with either an anti-GHRH antibody (14) (1:5,000 dilution) or anti–neuropeptide Y (NPY) antibody (15) (1:5,000 dilution) for 24 hours at room temperature. Immunostaining was visualized using the Vector SG substrate kit (Vector Laboratories).
Animal preparations and general protocol.
Tg and control rats were housed in air-conditioned animal quarters, with the lights on between 0800 hours and 2000 hours, and were given rat lab chow and water ad libitum. Rats (8 weeks old) were anesthetized with pentobarbital (50 mg/kg intraperitoneally), and a catheter was inserted into the right jugular vein.
Five Tg and five control rats were randomly injected intravenously with KP-102, a GHS that is also known as GHRP-2 (1 μg/kg; Kaken Pharmaceutical Co., Tokyo, Japan). Blood samples were collected at 0, 5, 10, 15, 30, and 60 minutes after injection. At the end of the experiment, plasma aliquots were assayed for rat GH with a radioimmunoassay.
To study the GH secretory pattern, rats were anesthetized and provided with an indwelling right atrial cannula 5 days prior to the study. Serial blood specimens (20 μl) were withdrawn via the cannula every 10 minutes with an automatic blood sampling device described by Clark and colleagues (16). Blood was collected from 0800 hours to 1600 hours. Each blood specimen was automatically diluted with heparinized saline (1:5) and assayed directly for rat GH. For the experiments on food intake, rats (6 weeks old) were anesthetized with pentobarbital, and a polyethylene guide cannula was implanted into the right lateral ventricle as previously described (17, 18). Five days after the operation, the animals were placed individually in metabolic cages and allowed free access to rat lab chow and water. All experiments were conducted between 1300 hours and 1500 hours. KP-102 or saline was administered intracerebroventricularly in a volume of 2 μl over 3 minutes, and food intake during the following 2 hours was measured. Daily food consumption was measured from weaning to 12 weeks of age in a cage (30 cm × 30 cm, 38 cm in height) equipped with a complete automatic feeding system (PAW 2000; Toyo Industry Co., Tokyo, Japan) connected to a computer.
Hormone assays.
Blood was obtained by decapitation early in the morning. GH levels were measured with a radioimmunoassay using materials supplied by NIDDK. Prolactin levels were measured with a rat prolactin assay system (Amersham Pharmacia Biotech). Insulin-like growth factor-I (IGF-I) levels were determined with a radioimmunoassay kit (Diagnostic Systems Laboratories Inc., Webster, Texas, USA) after extraction. Plasma ghrelin concentrations were measured using a ghrelin EIA kit (Phoenix Pharmaceuticals Inc., Belmont, California, USA) after extraction with Sep-Pak Plus C18 (Waters Corp., Milford, Massachusetts, USA).
Statistical analysis.
All values were expressed as mean ± SEM. Statistical analysis was performed with ANOVA followed by the Fisher least significant difference test.
Results
Generation of Tg rats.
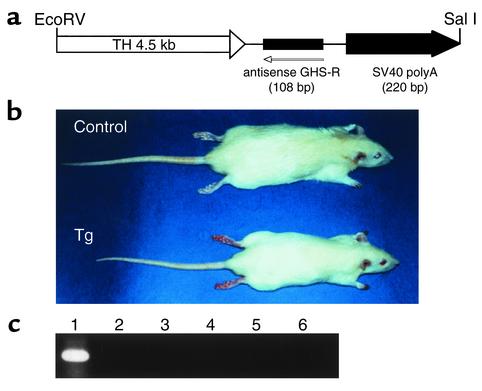
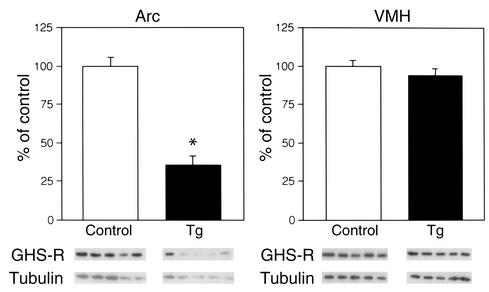
We developed Tg rats that express an antisense RNA for the GHS-R gene under the control of the TH promoter and have reduced levels of GHS-R protein (Figure 1a). The antisense construct was designed to be specific for the region around the initiation codon of GHS-R. Transcription of the antisense sequence was driven by the TH promoter, which usually exhibits activity in GHRH-containing neurons in the Arc (9, 19, 20). Fourteen Tg founder rats were identified using PCR analysis and then crossed with wild-type slc:SD rats. RT-PCR analysis of the hypothalamus showed higher expression of the antisense mRNA in two lines (termed 3-4 and 9-4) than in the other lines, and these two lines were selected to generate homozygous rats. Hemizygous Tg rats of the two lines were the same size, but were significantly smaller than the non-Tg control rats. Since homozygous rats of the 9-4 line were successfully established first, the 9-4 line was used for the analysis. The homozygous Tg rats were smaller and leaner than the control rats (Figure 1b). Expression of the antisense mRNA was detected in the hypothalamus using RT-PCR, whereas there was no detectable antisense mRNA in the heart, liver, kidney, testis, and pituitary of Tg rats (Figure 1c). To examine whether the antisense mRNA is in fact in the TH neurons in the hypothalamus of Tg rats, double-label in situ hybridization was performed. The antisense mRNA was detected in all the catecholaminergic neurons, whereas no ectopic expression was observed in any brain region of the Tg rats (data not shown). In the Arc, almost all the TH neurons had the antisense mRNA (Figure 2). There was no detectable antisense mRNA in the hypothalamus of control rats (data not shown). We compared GHS-R protein levels in the Arc and ventromedial nucleus of the Tg rats with those in the same nuclei of control rats using Western blot analysis. A decrease in the levels of GHS-R protein was seen in the Arc of the Tg rats, whereas there was no significant difference between Tg and control rats in the levels of GHS-R protein in the ventromedial nucleus (Figure 3).
Figure 1.
Generation of Tg rats expressing an antisense GHS-R gene. (a) Schematic structure of the antisense GHS-R transgene. (b) Gross appearance of a 6-week-old Tg rat and its non-Tg littermate. (c) Expression of the antisense GHS-R gene in Tg rats. Lane 1, hypothalamus; lane 2, heart; lane 3, liver; lane 4, kidney; lane 5, testis; lane 6, pituitary.
Figure 2.
In situ hybridization of GHS-R antisense mRNA and TH mRNA in the Arc of Tg rats. (a) Low magnification (scale bar: 60 μm). (b) High magnification (scale bar: 6 μm). Almost all the TH neurons (purple) express GHS-R antisense mRNA (blue). Cells with double staining are indicated by arrows. 3V, third ventricle.
Figure 3.
Western blot analysis of GHS-R protein in the hypothalamic nuclei of Tg rats. The levels of GHS-R protein were corrected for β-tubulin levels. Data is presented as mean ± SE for five 8-week-old rats. *P < 0.05 versus control rats. VMH, ventromedial nucleus of the hypothalamus.
Immunocytochemistry.
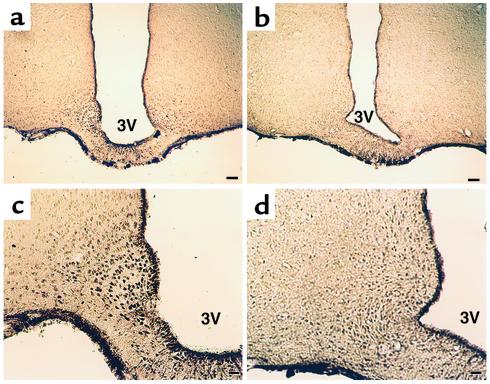
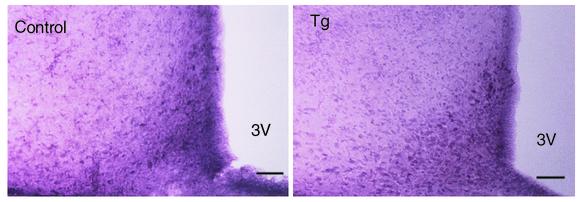
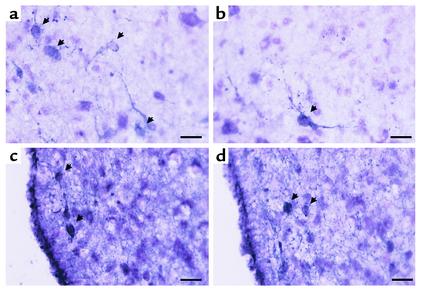
Immunocytochemistry for GHS-R demonstrated that the number of GHS-R–positive neurons was remarkably decreased in the Arc of Tg rats compared with control rats (Figure 4). GHS-R–like immunoreactivity was completely eliminated by addition of the synthetic GHS-R fragment (amino acid residues 248–260) used to raise the antiserum (data not shown) (13). The distribution of TH-immunoreactive cell bodies and fibers was similar between Tg and control rats (Figure 5).
Figure 4.
Immunocytochemistry of GHS-R protein in the Arc of control (a and c) and Tg (b and d) rats. (a and b) Low magnification (scale bar: 60 μm). (c and d) High magnification (scale bar: 24 μm).
Figure 5.
Immunocytochemistry for TH in control and Tg rats. Scale bars: 40 μm.
Double-labeling immunocytochemistry showed that many GHRH-immunoreactive neurons in the Arc of control rats contained GHS-R–like immunoreactivity, whereas there were only a few GHRH-immunoreactive neurons that also had GHS-R–like immunoreactivity in Tg rats (Figure 6, a and b). Some, but not all, NPY-immunoreactive neurons in the Arc of both control and Tg rats contained GHS-R–like immunoreactivity (Figure 6, c and d). There were several NPY-immunoreactive neurons in the Arc of both control and Tg rats that seemed to lack GHS-R–like immunoreactivity.
Figure 6.
Double-labeling immunocytochemistry for GHS-R and either GHRH (a and b) or NPY (c and d). (a and b) Double-labeling immunocytochemistry for GHS-R (purple staining) and GHRH (gray staining) in control (a) and Tg (b) rats. Cells with double staining are indicated by arrows. (c and d) Double-labeling immunocytochemistry for GHS-R (purple staining) and NPY (gray staining) in control (c) and Tg (d) rats. Cells with double staining are indicated by arrows. Scale bars: 10 μm.
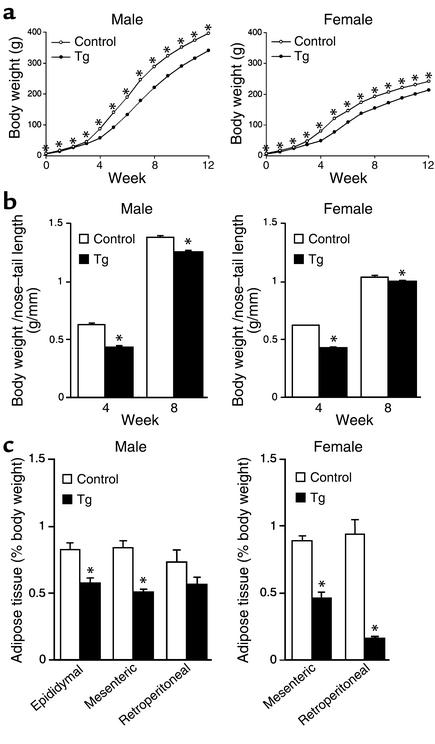
Body weight and body fat of Tg rats.
Tg rats were significantly smaller at birth, and a significant difference in body weight between Tg and control rats was continuously observed throughout the first 12 weeks after birth (Figure 7a). Tg rats had lower body weight/nose-tail length ratios, indicating that Tg rats were lean compared with control rats (Figure 7b). Tg rats had less adipose tissue than did control rats (Figure 7c). In male rats, significant differences in epididymal and mesenteric fat as percentage of body weight were found between the two groups (Figure 7c). Also, the percentages of mesenteric and retroperitoneal fat tissues were significantly decreased in Tg female rats compared with those in the control female rats (Figure 7c).
Figure 7.
Characterization of Tg rats. (a) Body weight curves. Each data point represents mean ± SEM of 8–25 rats (the error bars are smaller than the symbols for many of the data points). *P < 0.05 versus control rats. (b) Body weight/nose-tail length ratios at 4 and 8 weeks of age. Data are presented as mean ± SEM of six rats. *P < 0.05 versus control rats. (c) Adipose tissue (epididymal, mesenteric, and retroperitoneal) weight as a percentage of body weight of 8-week-old Tg rats. Columns represent the mean ± SEM of six rats. *P < 0.05 versus control rats.
GH secretory pattern and plasma IGF-I, ghrelin, and prolactin concentrations in Tg rats.
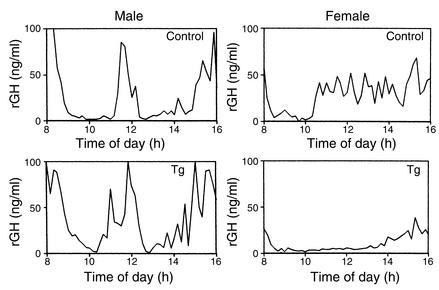
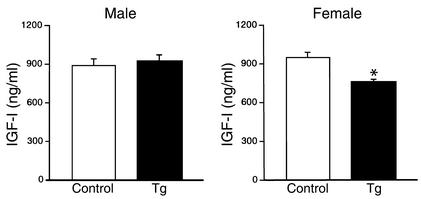
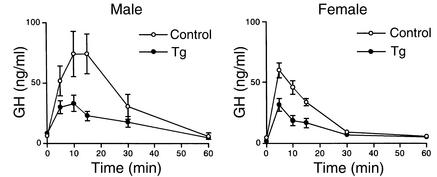
To identify GH pulses and baseline levels, data obtained from each rat were analyzed with the PULSAR computer program developed by Merriam and Wachter (21). The usual high bursts of pulsatile GH secretion were observed in male Tg rats (Figure 8), and there was no significant difference in pulse frequency and baseline concentrations between Tg and control male rats. On the other hand, while the blood GH concentrations of control female rats showed irregular small fluctuations and high baseline levels, Tg female rats showed low baseline concentrations and few pulses (Figure 8). Baseline GH concentrations in female Tg rats (9.3 ± 0.69 ng/ml) were significantly reduced compared with those in control female rats (23.5 ± 2.4 ng/ml, P < 0.05), and also, pulse frequency measured over a period of 8 hours was significantly decreased in female Tg rats (2.7 ± 0.25) compared with the control female rats (8.0 ± 0.1, P < 0.05). The plasma IGF-I levels were significantly lower in female Tg rats than in control female rats, whereas there was no significant difference in plasma IGF-I levels between Tg and control male rats (Figure 9). Plasma ghrelin levels were similar between Tg and control male rats (Tg, 350 ± 74 pg/ml; control, 198 ± 32 pg/ml). Also, there was no significant difference in plasma prolactin levels between Tg and control male rats (Tg, 3.6 ± 1.9 ng/ml; control, 7.4 ± 1.6 ng/ml).
Figure 8.
Representative blood GH profiles of Tg and control rats. rGH, rat GH.
Figure 9.
Plasma IGF-I concentrations in Tg rats. Data are presented as mean ± SEM of six rats. *P < 0.05 versus control rats.
Effect of a GHS on GH release in Tg rats.
In both male and female rats, the response of GH to KP-102 in Tg rats was significantly lower than that in control rats (Figure 10). The area under the GH curve was significantly smaller in Tg rats than in control rats (data not shown).
Figure 10.
Effect of KP-102 (1 μg/kg) on GH secretion in Tg rats. The data are expressed as the mean ± SEM of five rats.
Food intake in Tg rats.
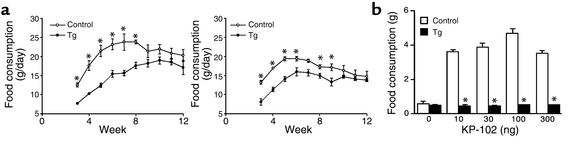
Male Tg rats ate less than age-matched control male rats ate from weaning to 8 weeks of age (Figure 11a). Similarly, the food consumption of female Tg rats was less than that of age-matched control female rats at 3, 4, 5, 6, 8, and 9 weeks of age (Figure 11a). As previously reported by our group, ICV injection of KP-102 significantly and dose-dependently increased food intake in normal control rats (2, 17, 18), whereas the effect of KP-102 on food intake was completely abolished in Tg rats (Figure 11b); even the highest dose of KP-102 (300 ng) did not increase food intake in Tg rats.
Figure 11.
Food intake in Tg rats. (a) Daily food intake. *P < 0.05 versus control rats. Data are expressed as the mean ± SEM of five rats. (b) Effect of KP-102 (10, 30, 100, and 300 ng) on food intake. The data are expressed as the mean ± SEM of ten rats. *P < 0.05 versus control rats.
Discussion
The ability to delete a gene of interest in a tissue-specific manner is a considerable advantage in studying the function of the gene. There are successful examples of the use of antisense mRNA in Tg animals for tissue-specific inhibition of target gene expression (22–24). In the present study, antisense GHS-R mRNA fused with the rat TH gene promoter (11) was used to disrupt the function of GHS-R in the hypothalamus, especially in the Arc. In fact, the expression of the antisense GHS-R mRNA was detected in almost all the TH neurons in the Arc of Tg rats, while no ectopic expression of the antisense mRNA was detected in neurons other than the TH neurons or peripheral tissues. Based on Western blot analysis and immunocytochemistry, GHS-R protein levels were decreased in the Arc of Tg rats compared with those of control rats, whereas there was no significant difference in GHS-R protein levels in the ventromedial nucleus between Tg and control rats. The distribution of TH in the Arc was not altered in Tg rats. These data indicate that the amount of antisense GHS-R mRNA accumulated was sufficient to suppress the synthesis of GHS-R protein in the Arc.
GH secretion has been shown to be regulated by two antagonistic hypothalamic peptides: GHRH, which stimulates, and somatostatin, which inhibits GH secretion from the pituitary. GHRH-containing neurons in the Arc have been proposed to be one of the central targets of ghrelin/GHS in stimulating GH secretion (10, 25, 26). In fact, the results of the present immunocytochemical study showed that many GHRH-containing neurons in the Arc of control rats coexpressed GHS-R. As expected from the finding that the majority of the GHRH neurons in the Arc also express TH (9, 19, 20), double-staining immunocytochemistry showed that only a few GHRH neurons in the Arc had GHS-R–like immunoreactivity in Tg rats. Therefore, the reduced GH response to KP-102 in Tg rats is probably due to the reduction of GHS-R in GHRH neurons in the Arc.
In our study, pulsatile GH secretion was preserved in male Tg rats, while GH secretion and plasma IGF-I levels were reduced in female Tg rats. Normal male rats show a regular and pronounced episodic pattern of GH secretion with extremely low basal secretion that is regulated by the cyclic rhythms of GHRH and somatostatin release (27). On the other hand, since normal female rats show a more continuous and irregular pattern of GH secretion with higher baseline levels than those of normal male rats, it appears that somatostatin output is noncyclical and rather low, and that GHRH plays a dominant role in GH secretion in female rats (27). Since somatostatin neurons do not contain TH (28), they should be unaffected in our Tg rats. Furthermore, the absence of GHS actions on somatostatin tone has been suggested (29). Therefore, the divergence of GH secretion between male and female Tg rats may be explained by the reduction of GHS-R in GHRH neurons in the Arc. It seems that in female rats, an endogenous ligand such as ghrelin plays a more important role in the regulation of GH secretion by stimulating GHRH neurons through GHS-R. The possibility that deletion of GHS-R in GHRH-containing neurons reduces GH secretion remains to be tested.
Recently, it has been reported that ghrelin and GHSs stimulate food intake in freely feeding rats (6–8, 30). However, the mechanism by which ghrelin/GHS promotes food intake is not fully understood. Our previous observations suggest that KP-102 and GHRH stimulate food intake via different mechanisms, and that GHRH neurons in the Arc are probably not involved in the KP-102–induced stimulation of food intake (2). NPY-containing neurons participate in the stimulatory mechanism for food intake (31, 32). Therefore, it is assumed that NPY is involved in the stimulatory effect of ghrelin/GHS on feeding, because half of the neurons expressing c-fos mRNA after GHS injection contain NPY (25). On the other hand, a significant increase in food intake in response to ghrelin is found in NPY-deficient mice, indicating that the presence of NPY is not obligatory for stimulation of food intake (8). In NPY-deficient mice, agouti-related protein (AgRP), which also stimulates feeding, may play an important role in food intake in place of NPY, since central administration of ghrelin increases AgRP mRNA (33). In the Tg rats described here, the stimulatory effect of KP-102 on feeding behavior was abolished. GHS-R–like immunoreactivity on NPY neurons was preserved in the Arc of Tg rats, which agrees with the report that most of the NPY neurons in the Arc do not contain TH (34). Therefore, KP-102 acts on unknown but TH-positive neurons possessing GHS-R, and these neurons may be responsible for the feeding effect of ghrelin/GHS. This possibility is supported by findings that GHS induces c-fos expression in cells other than GHRH or NPY neurons (25, 35). It is possible that KP-102 stimulates NPY neurons indirectly. In fact, at the electron microscopic level, direct appositions between TH- and NPY-immunoreactive structures have been observed in the Arc, suggesting direct interactions between these two systems (36).
Ghrelin/GHS induces a state of positive energy balance and body weight gain by promoting food intake and reducing fat utilization (8, 30). Tg rats showed significantly reduced daily food intake compared with age-matched control rats. They also had significantly lower body weight and body weight/nose-tail ratios, and less adipose tissue than control rats had. These findings could be the consequence of a defect in the hypothalamic action of endogenous ghrelin, which causes body weight gain by increasing food intake and decreasing fat utilization (8). How ghrelin/GHS regulates energy balance is still unknown. GH is well-known to have metabolic actions and to be involved in lipid metabolism (37). However, it appears unlikely that the induction of a positive energy balance by ghrelin/GHS is caused by its ability to stimulate GH secretion, because ghrelin-induced adiposity is not mimicked by GH (8). ICV administration of ghrelin suppressed serum concentrations of thyroid-stimulating hormone in rats (7). Considering that thyroid hormone increases total energy expenditure, it is possible that ghrelin/GHS regulates energy balance partly by suppressing thyroid-stimulating hormone. Further study is needed to clarify this issue.
The plasma levels of ghrelin were not significantly increased in Tg rats. The reduced GHS-R expression in the Arc did not seem to induce a compensatory secretion of ghrelin. The absence of a significant change in plasma prolactin levels in Tg rats is consistent with the intactness of TH-containing neurons in the Arc.
In summary, we have generated Tg rats in which the expression of GHS-R protein was selectively attenuated in the Arc. Our data suggest that the endogenous ghrelin/GHS-R system in the Arc regulates GH secretion, food intake, and adiposity. The Tg rat described here could be a useful animal model for investigating the physiological function of the ghrelin/GHS-R system in the hypothalamus.
Acknowledgments
We thank M. Ashizawa, T. Shimizu, N. Yamauchi, S. Inada, and M. Iketani for their technical assistance. This work was supported in part by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology, the Japanese Ministry of Health, Labour and Welfare, and the Foundation for Growth Science.
References
- 1.Smith RG, et al. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18:621–645. doi: 10.1210/edrv.18.5.0316. [DOI] [PubMed] [Google Scholar]
- 2.Okada K, et al. Intracerebroventricular administration of the growth hormone-releasing peptide KP-102 increases food intake in free-feeding rats. Endocrinology. 1996;137:5155–5158. doi: 10.1210/endo.137.11.8895390. [DOI] [PubMed] [Google Scholar]
- 3.Howard AD, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 4.Guan XM, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 5.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 6.Nakazato M, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 7.Wren AM, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 8.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 9.Meister B, et al. Coexistence of tyrosine hydroxylase and growth hormone-releasing factor in a subpopulation of tubero-infundibular neurons of the rat. Neuroendocrinology. 1986;42:237–247. doi: 10.1159/000124446. [DOI] [PubMed] [Google Scholar]
- 10.Tannenbaum GS, Lapointe M, Beaudet A, Howard AD. Expression of growth hormone secretagogue-receptors by growth hormone-releasing hormone neurons in the mediobasal hypothalamus. Endocrinology. 1998;139:4420–4423. doi: 10.1210/endo.139.10.6330. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee SA, Roffler-Tarlov S, Szabo M, Frohman L, Chikaraishi DM. DNA regulatory sequences of the rat tyrosine hydroxylase gene direct correct catecholaminergic cell-type specificity of a human growth hormone reporter in the CNS of transgenic mice causing a dwarf phenotype. Brain Res Mol Brain Res. 1994;24:89–106. doi: 10.1016/0169-328x(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 12.Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- 13.Shuto Y, et al. Generation of polyclonal antiserum against the growth hormone secretagogue receptor (GHS-R): evidence that the GHS-R exists in the hypothalamus, pituitary and stomach of rats. Life Sci. 2001;68:991–996. doi: 10.1016/s0024-3205(00)01001-8. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi N, Shibasaki T, Ling N, Demura H. In vitro release of growth hormone-releasing factor (GRF) from the hypothalamus: somatostatin inhibits GRF release. Regul Pept. 1991;33:71–78. doi: 10.1016/0167-0115(91)90016-a. [DOI] [PubMed] [Google Scholar]
- 15.Shibasaki T, Oda T, Imaki T, Ling N, Demura H. Injection of anti-neuropeptide Y gamma-globulin into the hypothalamic paraventricular nucleus decreases food intake in rats. Brain Res. 1993;601:313–316. doi: 10.1016/0006-8993(93)91727-a. [DOI] [PubMed] [Google Scholar]
- 16.Clark RG, Jansson JO, Isaksson O, Robinson IC. Intravenous growth hormone: growth responses to patterned infusions in hypophysectomized rats. J Endocrinol. 1985;104:53–61. doi: 10.1677/joe.0.1040053. [DOI] [PubMed] [Google Scholar]
- 17.Shibasaki T, et al. The growth hormone secretagogue KP-102-induced stimulation of food intake is modified by fasting, restraint stress, and somatostatin in rats. Neurosci Lett. 1998;255:9–12. doi: 10.1016/s0304-3940(98)00695-8. [DOI] [PubMed] [Google Scholar]
- 18.Kuriyama H, Hotta M, Wakabayashi I, Shibasaki T. A 6-day intracerebroventricular infusion of the growth hormone-releasing peptide KP-102 stimulates food intake in both non-stressed and intermittently-stressed rats. Neurosci Lett. 2000;282:109–112. doi: 10.1016/s0304-3940(00)00882-x. [DOI] [PubMed] [Google Scholar]
- 19.Niimi M, Takahara J, Sato M, Kawanishi K. Identification of dopamine and growth hormone-releasing factor-containing neurons projecting to the median eminence of the rat by combined retrograde tracing and immunohistochemistry. Neuroendocrinology. 1992;55:92–96. doi: 10.1159/000126101. [DOI] [PubMed] [Google Scholar]
- 20.Zoli M, Agnati LF, Tinner B, Steinbusch HW, Fuxe K. Distribution of dopamine-immunoreactive neurons and their relationships to transmitter and hypothalamic hormone-immunoreactive neuronal systems in the rat mediobasal hypothalamus. A morphometric and microdensitometric analysis. J Chem Neuroanat. 1993;6:293–310. doi: 10.1016/0891-0618(93)90034-2. [DOI] [PubMed] [Google Scholar]
- 21.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion in stalk-sectioned rats. Am J Physiol. 1982;243:E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- 22.Moxham CM, Hod Y, Malbon CC. Induction of G alpha i2-specific antisense RNA in vivo inhibits neonatal growth. Science. 1993;260:991–995. doi: 10.1126/science.8493537. [DOI] [PubMed] [Google Scholar]
- 23.Pepin M-C, Pothier F, Barden N. Impaired type II glucocorticoid-receptor function in mice bearing antisense RNA transgene. Nature. 1992;355:725–728. doi: 10.1038/355725a0. [DOI] [PubMed] [Google Scholar]
- 24.Manshouri T, et al. Downregulation of RARα in mice by antisense transgene leads to a compensatory increase in RARβ and RARγ and development of lymphoma. Blood. 1997;89:2507–2515. [PubMed] [Google Scholar]
- 25.Dickson SL, Luckman SM. Induction of c-fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology. 1997;138:771–777. doi: 10.1210/endo.138.2.4907. [DOI] [PubMed] [Google Scholar]
- 26.Kamegai J, et al. Growth hormone-dependent regulation of pituitary GH secretagogue receptor (GHS-R) mRNA levels in the spontaneous dwarf rat. Neuroendocrinology. 1998;68:312–318. doi: 10.1159/000054379. [DOI] [PubMed] [Google Scholar]
- 27.Robinson ICAF. The growth hormone secretory pattern: a response to neuroendocrine signals. Acta Paediatr Scand Suppl. 1991;372:70–78. doi: 10.1111/j.1651-2227.1991.tb17975.x. [DOI] [PubMed] [Google Scholar]
- 28.Everitt BJ, et al. The hypothalamic arcuate nucleus-median eminence complex: immunohistochemistry of transmitters, peptides and DARPP-32 with special reference to coexistence in dopamine neurons. Brain Res. 1986;396:97–155. doi: 10.1016/s0006-8993(86)80192-5. [DOI] [PubMed] [Google Scholar]
- 29.Tannenbaum GS, Bowers CY. Interactions of growth hormone secretagogues and growth hormone-releasing hormone/somatostatin. Endocrine. 2001;14:21–27. doi: 10.1385/ENDO:14:1:021. [DOI] [PubMed] [Google Scholar]
- 30.Asakawa A, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 31.Kalra SP, Horvath TL. Neuroendocrine interactions between galanin, opioids, and neuropeptide Y in the control of reproduction and appetite. Ann NY Acad Sci. 1998;863:236–240. doi: 10.1111/j.1749-6632.1998.tb10698.x. [DOI] [PubMed] [Google Scholar]
- 32.Gehlert DR. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides. 1999;33:329–338. doi: 10.1054/npep.1999.0057. [DOI] [PubMed] [Google Scholar]
- 33.Kamegai J, et al. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797–4800. doi: 10.1210/endo.141.12.7920. [DOI] [PubMed] [Google Scholar]
- 34.Everitt BJ, et al. Differential co-existence of neuropeptide Y (NPY)-like immunoreactivity with catecholamines in the central nervous system of the rat. Neuroscience. 1984;11:443–462. doi: 10.1016/0306-4522(84)90036-8. [DOI] [PubMed] [Google Scholar]
- 35.Kamegai J, Hasegawa O, Minami S, Sugihara H, Wakabayashi I. The growth hormone-releasing peptide KP-102 induces c-fos expression in the arcuate nucleus. Brain Res Mol Brain Res. 1996;39:153–159. doi: 10.1016/0169-328x(96)00020-4. [DOI] [PubMed] [Google Scholar]
- 36.Guy J, Pelletier G. Neuronal interactions between neuropeptide Y (NPY) and catecholaminergic systems in the rat arcuate nucleus as shown by dual immunocytochemistry. Peptides. 1988;9:567–570. doi: 10.1016/0196-9781(88)90165-9. [DOI] [PubMed] [Google Scholar]
- 37.Thorner, M.O., et al. 1998. Growth hormone actions. In Williams textbook of endocrinology. 9th edition. J.D. Wilson, D.W. Foster, H.M. Kronenberg, and P.R. Larsen, editors. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 260–263.