Abstract
NF-κB essential modifier (NEMO), also known as IKK-γ, is a member of the I-κB kinase complex responsible for phosphorylating I-κB, allowing the release and activation of NF-κB. Boys with an expressed NEMO mutation have an X-linked syndrome characterized by hypohidrotic ectodermal dysplasia with immune deficiency (HED-ID). The immunophenotype resulting from NEMO mutation is highly variable, with deficits in both T and B cell responses. We evaluated three patients with NEMO mutations (L153R, Q403X, and C417R) and HED-ID who had evidence of defective CD40 signaling. All three patients had normal percentages of peripheral blood NK cells, but impaired NK cell cytotoxic activity. This was not due to a generalized defect in cytotoxicity because antibody-dependent cellular cytotoxicity was intact. This abnormality was partially reversed by in vitro addition of IL-2, which was also able to induce NF-κB activation. In one patient with recurrent cytomegalovirus infections, administration of IL-2 partially corrected the NK cell killing deficit. These data suggest that NEMO participates in signaling pathways leading to NK cell cytotoxicity and that IL-2 can activate NF-κB and partially overcome the NK cell defect in patients with NEMO mutations.
Introduction
Hypohidrotic ectodermal dysplasia (HED) is a rare syndrome associated with aberrant development of the hair, teeth, and eccrine sweat glands (1). The majority of cases of HED are X-linked and are due to mutations of the gene coding for ectodysplasin-A, a member of the TNF superfamily (2). A minority of cases are autosomal recessive and are due to mutations in the ectodysplasin-A receptor, which is the homologue of the murine downless gene (3), or a mutation in the Edar-associated death domain adapter protein, which is involved in downstream signaling from the ectodysplasin-A receptor (4). A small subset of X-linked cases of HED are associated with immunodeficiency, characterized by susceptibility to mycobacterial and streptococcal infections, dys-γ-globulinemia (with decreased IgG and decreased or elevated IgM and IgA), poor polysaccharide-specific antibody responses, and depressed antigen-specific lymphocyte proliferation (1, 5–9).
Recent studies have demonstrated HED with immunodeficiency (HED-ID) to be associated with mutations in the gene coding for NF-κB essential modifier (NEMO), also known as I-κB kinase (IKK)-γ (7–9). The NEMO gene consists of ten exons and codes for a scaffold protein that binds IKK-α and IKK-β and is essential for forming a functional IKK complex (10). Signaling by a variety of cell surface receptors activates the IKK complex, rendering it capable of phosphorylating I-κBα, which allows translocation of NF-κB to the nucleus where it participates in transcriptional regulation (11). The majority of patients with HED-ID who have been evaluated have a point mutation in the C-terminal portion of the NEMO gene that encodes a zinc finger domain believed to be an important modulator for upstream activators (7–9). These point mutations are associated with reduced IKK function (8, 9). Incontinentia pigmenti, an HED-like syndrome in females that is most prominently characterized by skin hyperpigmentation and dermal scarring, is also due to mutations in one of the two NEMO alleles (12). The NEMO mutations found in women with incontinentia pigmenti involve significant frameshifts and large deletions that are associated with complete loss of IKK function in affected cells, making offspring who carry the mutation die in utero (12, 13).
Natural killer (NK) cells are lymphocytes that lack surface expression of T cell receptor/CD3 and Ig that are important in defense against certain infectious disease. Complete absence of NK cells has been associated with susceptibility to infection with herpesviruses (14, 15) and other viral pathogens (16). A number of receptors on NK cells are capable of mediating cytotoxicity toward tumor cells or virally infected cells without prior sensitization, resulting in the exocytosis of lytic granules (17). This is known as NK cell cytotoxicity, or natural or spontaneous cytotoxicity. Examples of molecules involved in initiating NK cell cytotoxicity include activating killer cell immunoglobulin-like receptors (KIRs) and β integrins. The control of this activity is ensured via dominant inhibition by ligation of inhibitory KIRs that recognize a variety of MHC class I molecules. NK cells are also the major mediators of antibody-dependent cellular cytotoxicity (ADCC), which is initiated by ligation of their surface FcγIII receptor, CD16.
Ligation of cell surface NK cell–activating receptors results in activation of cytoplasmic protein kinases, leading to cytolytic activity. Although many kinases are common to both ADCC and NK cell cytotoxicity pathways, there appear to be noticeable differences (18). Specifically, NK cell cytotoxic activity relies upon phosphatidylinositol 3-kinase (PI3K) and proline-rich tyrosine kinase-2 (19–22). Naturally occurring human mutations delineating the intracellular distinctions between NK cell cytotoxicity and ADCC have not been described. Thus, the relative usefulness of each of these two individual NK cell activities in the control of human disease is unknown.
The studies presented here demonstrate deficiency of NK cell cytotoxicity, but normal ADCC in boys with HED-ID and mutations in NEMO. These results suggest a role for NEMO in NK cell cytotoxicity.
Methods
Patients, controls, and treatments.
Three patients with HED determined by clinical appearance and skin biopsy demonstrating absent eccrine sweat glands, were evaluated with parental consent/patient assent. All studies were approved by the Children’s Hospital Committee on Clinical Investigation (CH-CCI). Blood samples were obtained from patients or healthy adult and adolescent volunteer control donors who had no recent history of illness and were tested on the same or the following day blood was drawn. Disease controls 1 and 2 were a 4-year-old with recurrent herpes simplex encephalitis and a 12-year-old with disseminated varicella. Neither disease control had a defined immune defect. In vivo IL-2 (aldesleukin; Chiron Corp., Emeryville, California, USA) treatment was a continuous infusion of 1 × 106 U/m2 for 5 days. Separate parental consent and CH-CCI approval was obtained for therapy.
Patient 1 had Listeria monocytogenes sepsis, Streptococcus bovis meningitis, cytomegalovirus (CMV) sepsis, and recurrent CMV colitis. After his initial CMV episode, CMV-specific IgM was 309 U (>50 is consistent with a primary response), and during his second CMV recurrence, CMV-specific IgG was 4.28 IU/ml (>1.09 is consistent with a protective response). Total IgG ranged from 99 to 374 mg/dl (normal range for this patient’s age is 500–1,500); IgM was 14–59 mg/dl (normal: 30–100), IgA was 17–435 mg/dl (normal: 10–75), and IgE was 1–3 U/ml (normal: 4–60). Detectable titer against diphtheria and tetanus, as well as in vitro proliferation to diphtheria and tetanus were present after two immunizations, and delayed-type hypersensitivity reaction to Candida was positive. Sequence evaluation of the gene coding for ectodysplasin-A was unremarkable. Our evaluations of this patient were performed when he was between 14 and 24 months of age.
Patient 2 had sinusitis, pneumonia, gram-positive bacteremia, and oral herpetic lesions. At age 7 he developed cutaneous granulomas and had Mycobacterium avium-intracellulare isolated from blood, bone marrow, and skin. At presentation, IgG was 170 mg/dl (normal: 280–1,500), IgA was less than 10 mg/dl (normal: 16–50), and IgM was 14 mg/dl (normal: 15–70); he was treated with regular intravenous immunoglobulin therapy. He had normal mitogen-induced proliferation, and present but decreased specific antibody and proliferative responses. Our evaluations of this patient were performed when he was 17 years old.
Patient 3 had recurrent sinopulmonary infections, Staphylococcus aureus adenitis, and Klebsiella pneumoniae bacteremia. At 14 years of age he developed granulomatous chronic vertebral osteomyelitis from which Mycobacterium abscessus was isolated. Prior to starting intravenous immunoglobulin at age 2, this patient’s IgG was 116 mg/dl (normal: 300–1500), IgA was 326 mg/dl (normal: 30–150), and IgM was 56 mg/dl (normal: 20–100). Serum tetanus titer and delayed hypersensitivity were negative despite three immunizations. He had normal mitogen-induced lymphocyte proliferation but reduced antigen-specific responses. Our evaluations of this patient were performed when he was 16 years old.
Cell preparation and culture conditions.
PBMCs were obtained from fresh heparinized blood by density gradient centrifugation on Ficoll-Hypaque (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA). Cell populations were phenotyped by flow cytometry using a FACSCalibur flow cytometer and commercially available monoclonal antibodies conjugated directly to FITC or phycoerythrin (PE) (Becton Dickinson Immunocytometry Systems, San Jose, California, USA). Cell culture and stimulation assays were performed in RPMI 1640 containing 10% heat-inactivated FBS, 2 mmol/l L-glutamine, 50 μg/ml streptomycin, and 100 U/ml penicillin. For in vitro ligation of CD40, either anti-CD40 monoclonal antibody 626.1 (a gift of S.M. Fu, University of Virginia, Charlottesville, Virginia, USA) or soluble murine/human CD8:CD40L (sCD40L) with rat anti-mouse CD8 (obtained as described in ref. 23) were added to the cultures. B cell–enriched populations were prepared from PBMCs by the removal of T cells by rosetting (24). Enriched populations contained 85% B cells, 5% T cells, and 10% other cells. NK cell enrichment was performed using the Dynabeads NK cell negative selection system according to manufacturer recommendations (Dynal Biotech, Great Neck, New York, USA) and yielded greater than 90% CD56+/CD3– cells. Patient NK cells in total PBMCs were evaluated by flow cytometry using the standard clinical reagent consisting of CD56-PE, CD16-PE, and CD3-FITC, or by tricolor staining using CD56-PE, CD16-FITC, and CD3-CY5 (Becton Dickinson Immunocytometry Systems). Human recombinant IL-2 (Chiron Corp.) or IL-4 (R&D Systems Inc., Minneapolis, Minnesota, USA) was used as specified. Phytohemagglutinin (PHA; Murex Biotech Ltd., Dartford, United Kingdom) was used for stimulation of PBMCs at 2 μg/ml.
NEMO amplification and sequencing.
Total genomic DNA and RNA were extracted from fresh PBMCs, and cDNA was prepared by standard methods. Individual PCR primers for each exon of NEMO were designed from the GenBank sequence for the gene and used for amplification of genomic and cDNA sequences. Exon 4 was evaluated using the following primers: 5′-CAGTGCTGACAGGAAGTGGC-3′ for 5′ amplification and 5′-AACCCTGGAAGGGGTCTCCGGAG-3′ for 3′ amplification. Primers and approach for determination of exon 10 mutations were as described (7, 9). Sequences were obtained from both the 5′ and 3′ ends of PCR products.
CD40 functional assays.
For proliferation studies, cells were cultured at 1 × 106 cells/ml for 96 hours in the presence or absence of 5 ng/ml IL-4 and/or 5 μg/ml anti-CD40 monoclonal antibody with 3H-thymidine added for the final 18 hours. Cellular incorporation of 3H was assayed by water lysis and filter-binding, followed by liquid scintillation using a Wallac 1042 scintillation counter (Perkin-Elmer Inc., Boston, Massachusetts, USA). Induction of activation markers was performed by incubating 1 × 106 PBMCs/ml with 5 μg/ml anti-CD40 monoclonal antibody or media for 96 hours. Cells were washed twice and prepared for flow cytometric analysis using FITC-labeled anti-CD20 and either PE-labeled anti-CD23, or anti-CD54 (BD Pharmingen, San Diego, California, USA). Gated lymphocytes were analyzed for CD20, and positive events were evaluated for PE positivity. For IgE production, PBMCs were cultured at 1.5 × 106 cells/ml with or without 100 U/ml IL-4 and/or soluble murine CD8:CD40L for 14 days. Cell supernatants were harvested and assessed for IgE content by ELISA as described (25).
Electrophoretic mobility-shift assay.
Evaluation of NF-κB was performed on nuclear extracts as described (26). The NF-κB probe (Promega Corp., Madison, Wisconsin, USA) was end-labeled with γ-32P ATP (Perkin-Elmer Inc.). To evaluate CD40 function, nuclear extracts were prepared from B cell–enriched populations that were incubated for 30 minutes with sCD40L or medium. To determine the effect of IL-2, nuclear extracts were prepared from PBMCs that were incubated for 18 hours with 2 μg/ml PHA, rested in the absence of PHA for 5 hours, and then stimulated with 1,000 U/ml IL-2 for 5 hours. Radiographic signals were quantified by densitometry using NIH Image 1.62, with which signal found after stimulation was normalized to the unstimulated state.
Cytotoxicity assays.
Standard 4-hour 51Cr release assays were performed as described (16). PBMCs were used as effector cells. 51Cr-labeled K562 (an NK cell–sensitive erythroleukemia cell line), CL27A (an NK cell–resistant murine lymphoma cell line), or ZKBB (an NK cell–resistant human B lymphoblastoid cell line) were used as target cells at effector-to-target cell (E:T)ratios of 100:1, 50:1, 25:1, 12.5:1, 6.2:1, and 3.1:1. When enriched NK cells were evaluated, tenfold fewer effector cells were used. NK cell cytotoxicity is defined as lysis of K562 target cells. Individual assays were performed in duplicate or triplicate with 1 × 104 target cells in 96-well V-bottom plates. After 4 hours of incubation at 37°C, supernatants were assessed for free isotope, and percent specific lysis was calculated as 100 × (free isotope – spontaneously released isotope)/(total releasable isotope – spontaneously released isotope). Mean data were compared using the two-tailed Student t test. Lytic units were defined as the inverse of the number of lymphocytes required to mediate 20% lysis of K562 cells × 1 × 104. In vitro IL-2 stimulation was performed by adding 1,000 U/ml IL-2 at the start of the 4-hour cytotoxicity assay where indicated. ADCC was determined by the addition of CL27A mouse polyclonal antibody to CL27A, or monoclonal anti-CD20 (Rituximab; Genentech Inc., South San Francisco, California, USA) to ZKBB cells prior to combination of effector and target cells. Lysis of CL27A or ZKBB cells without antibody was measured with patient and control PBMCs and was consistently negative. Patient and respective control samples were always evaluated in parallel using the same antibody/target cell combination.
Results
Patients with HED-ID possess functionally significant NEMO mutations.
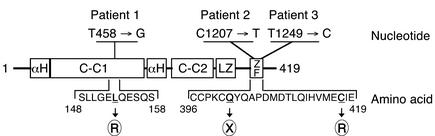
Individual exons of the NEMO gene were amplified by PCR. A single base substitution was detected in each of the three patients. Because NEMO is also present as a pseudogene containing exons 3–10, NEMO cDNA was also analyzed to confirm that the mutation was in the expressed NEMO gene. Patient 1 had a T458 → G point mutation in exon 4 that results in L153 → R substitution within the first coiled coil domain (Figure 1). Patient 2 had a C1207 → T point mutation in exon 10, resulting in a Q403 → X truncation of the zinc finger domain. Patient 3 had a T1249 → C point mutation in exon 10, causing a C417 → R substitution within the zinc finger domain. The mutations in patients 1 and 2 have not been previously described, whereas the mutation found in patient 3 has been reported in other individuals with HED-ID (7–9). None of these mutations had been found in previous analyses of at least 40 normal chromosomes (7, 9).
Figure 1.
NEMO mutations in patients with HED-ID. A schematic diagram of the human NEMO protein is shown with the individual domains labeled in boxes (αH, α-helix; C-C, coiled-coil; LZ, leucine zipper; ZF, zinc finger). Sequences containing amino acid substitutions in the three patients studied are displayed in brackets below the gene map. Arrows indicate positions of amino acid substitutions. The particular amino acid altered (underlined) and the substituted residues (circled) are shown. The specific nucleotide point mutation resulting in the missense amino acid is listed above the gene mutation.
CD40 is a member of the TNF receptor superfamily expressed on all B cells. CD40 ligation induces proliferation, CD23 and CD54 gene expression, and immunoglobulin class switching (23–25). Deficits in these CD40 signaling events have been demonstrated in patients with NEMO mutations (9). The functional significance of the mutations in our patients was evaluated by examining the response of their B cells to CD40 ligation. PBMCs from control donors typically demonstrated four- to eightfold induction of proliferation following CD40 ligation. In contrast, PBMCs from all three patients proliferated poorly or not at all in response to CD40 ligation (Figure 2a). Similar results were obtained in B cell–enriched populations (data not shown). The synergy between IL-4 and CD40 ligation in inducing proliferation observed in control PBMCs was preserved in PBMCs from two of the three patients with NEMO mutation. These results are consistent with those of Doffinger et al. (8).
Figure 2.
Functional consequences of CD40 ligation in PBMC cultures from patients with NEMO mutation. (a) Proliferation of PBMCs incubated with control medium, IL-4, anti-CD40, or IL-4 + anti-CD40 was determined by 3H incorporation. Black bars show proliferation in a control donor and white bars show proliferation in patients. Data are representative of two experiments each from each patient. (b) Surface expression of CD23 and CD54 was evaluated on CD20+ lymphocytes in representative controls (black bars) or patients (dark gray bars) and on anti-CD40–stimulated cells of controls (white bars) or patients (light gray bars). (c) IgE production was measured in supernatants of PBMC cultures from control donors (black bars) or patients (white bars) after incubation with medium, sCD40L, IL-4, or sCD40L + IL-4.
CD40-mediated upregulation of CD23 and CD54 surface expression in B cells was deficient in all three patients (Figure 2b) and was consistent with prior results (8, 9). IgE synthesis by PBMCs following stimulation with sCD40L + IL-4 was normal in patients 1 and 2, but was severely deficient in patient 3 (Figure 2c). Variability in the IgE response in patients with NEMO mutations has been previously reported (8).
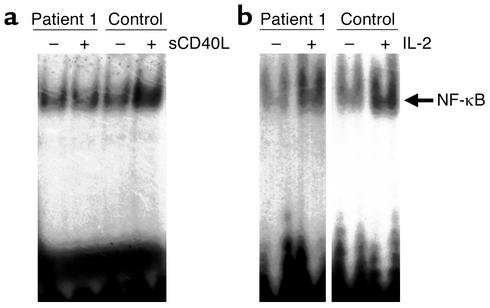
CD40-mediated activation of the NF-κB pathway has been demonstrated to be deficient in patients with NEMO mutations in exon 10, one of whom had the same mutation as our patient 3 (9). Because patient 1 had a novel exon 4 mutation, and because the only previously reported exon 4 mutation has not been functionally characterized, we examined CD40-mediated NF-κB activation in this patient. NF-κB activation was evaluated in nuclear extracts of enriched B cells by electrophoretic mobility-shift assay (EMSA) (Figure 3a). CD40 ligation resulted in increased NF-κB binding in control PBMCs (2.8-fold as quantitated by densitometry). In contrast, CD40 ligation did not cause increased NF-κB binding in the patient’s PBMCs, and there was no detectable difference between stimulated and unstimulated cells by densitometry.
Figure 3.
Evaluation of nuclear NF-κB level in cells from a patient with NEMO exon 4 mutation (patient 1). Nuclear extracts of enriched B cells from patient 1 or a control donor were evaluated by EMSA using a 32P-labeled NF-κB probe. (a) The effect of CD40 ligation on the level of nuclear NF-κB was determined by incubation of enriched B cells with control medium or sCD40L for 30 minutes prior to cell lysis. (b) The ability of IL-2 to induce nuclear levels of NF-κB was assessed in PBMCs by stimulation with PHA for 18 hours, followed by resting for 5 hours and subsequent incubation with IL-2 or media for 5 hours prior to lysis. Nuclear extracts were prepared and evaluated for NF-κB binding. The arrow points to the retained NF-κB probe; the large band at the bottom of the gel corresponds to free probe.
Taken together, these data suggest that the NEMO mutations in our patients are functionally significant.
Deficient NK cell cytotoxicity in patients with NEMO mutations.
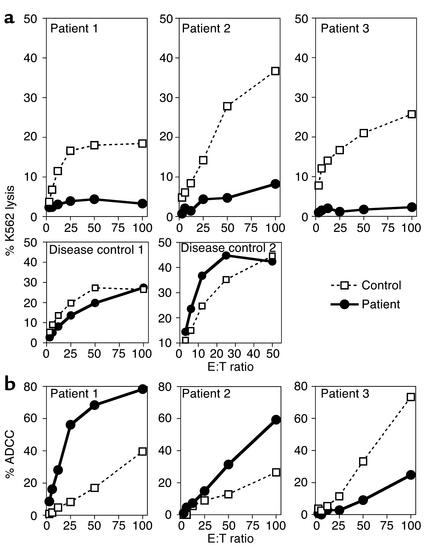
The importance of NK cell activity in defense against CMV infection has been established (14). The recurrence of symptomatic CMV disease in patient 1 in the face of an appropriate CMV-specific antibody response, and preserved proliferation of T cells in response to specific antigens, prompted us to examine NK activity in our patients. All patients had typical large granular lymphocytes as visualized by light microscopy. Flow cytometric analysis performed on multiple occasions revealed normal percentages of CD56+CD16+CD3– cells in all three patients. At the time of initial evaluation, patient 1 had 11% NK cells (patient lifetime range 5–33%), patient 2 had 6% (patient lifetime range 6–12%), and patient 3 had 3% (patient lifetime range 2–13%). NK cell cytotoxicity was evaluated by lysis of 51Cr-labeled K562 cells. All three patients were deficient in K562 killing compared with healthy controls (Figure 4a). Across multiple evaluations, the patients had a mean of 5.4% ± 2.7% K562 lysis at a 50:1 E:T ratio compared with 24.0% ± 3.8% for controls (P < 0.05). The deficiency of NK cell cytotoxic activity in these patients is unlikely to be due simply to persistent viral infection, because two patients with severe recurrent herpesvirus disease had normal K562 killing (Figure 4a). To ensure that there was no cellular inhibitor of NK cell cytotoxicity in the patients’ PBMCs, and to normalize for slight differences in NK cell percentages of PMBCs, NK cells enriched by negative selection from patient 1 were examined. Enriched NK cell populations consisted of more than 93% CD56+CD3– NK cells; of these, more than 85% expressed CD16. The NK cell cytotoxicity of purified NK cells from the patient was deficient, at 581 lytic units compared with 3,677 lytic units from the normal control.
Figure 4.
Deficient NK cell cytotoxicity in patients with HED-ID and NEMO mutation. (a) NK cell cytotoxicity was assessed in PBMCs from patients (circles) and controls (squares) by 51Cr-release assay with K562 target cells (top panels). Results are representative of a total of five experiments from patient 1 and two experiments each from patients 2 and 3. Patient and control samples were evaluated in parallel. Disease controls (bottom panels) are as described in Methods. (b) ADCC was measured in patients (circles) and controls (squares) using CL27A cells and antiserum (patients 1 and 3), or ZKBB cells and anti-CD20 monoclonal antibody (patient 2) as target cells. Lysis of 51Cr-labeled target cells was determined after 4 hours. Results are representative of three individual experiments for patient 1 and one experiment for patients 2 and 3. Lysis of target cells without added antibody was not detected. ADCC was always evaluated in parallel with K562 lysis.
To determine whether the deficiency of NK cell cytotoxicity in patients with NEMO mutations was due to a generalized defect in NK cell cytotoxic functions, ADCC was evaluated. ADCC was present at high levels in the patients’ peripheral blood (Figure 4b). No lysis of target cells occurred in the absence of added antiserum (data not shown). These data demonstrate that patients with NEMO mutation have a specific defect in NK cell cytotoxic activity.
Effects of IL-2 stimulation on NK cell cytotoxicity in patients with NEMO mutations.
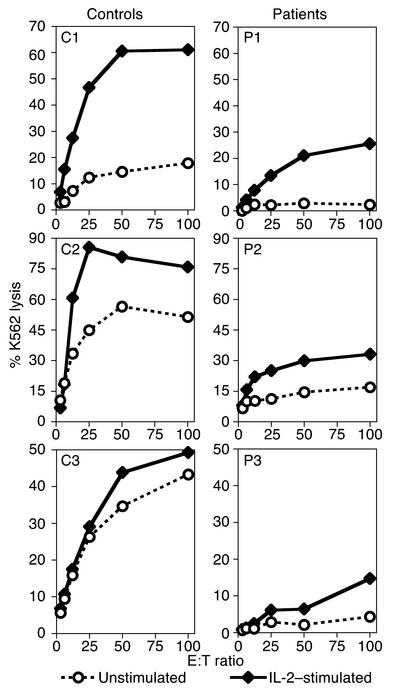
NK cell cytotoxic activity is known to be enhanced by a variety of cytokines. Among the most effective is IL-2, which has also been shown to induce NK activity in vitro and upon administration in vivo (27). To determine whether NK cell cytotoxicity in patients with NEMO mutations can be induced by IL-2, PBMCs were incubated with recombinant human IL-2 and lysis of K562 cells was measured. As expected, in vitro addition of IL-2 enhanced K562 killing in control PBMCs (Figure 5). At a 50:1 E:T ratio, there was a 2.3-fold ± 1.2-fold increase over baseline (n = 3). IL-2 induced K562 killing in PBMCs from all three NEMO patients. At a 50:1 E:T ratio, induction over baseline was greater than sevenfold in patient 1, 2.1-fold in patient 2, and threefold in patient 3 (Figure 5). However, IL-2–stimulated NK activity in the patients remained lower than that in controls. These results suggest that IL-2 can partially overcome the deficit in NK cell cytotoxicity in vitro in patients with NEMO mutations.
Figure 5.
In vitro IL-2 induction of NK cell cytotoxicity in PBMCs of patients with HED-ID and NEMO mutation. The effect of IL-2 on NK cell cytotoxic activity by PBMCs from control donors and patients was evaluated in parallel. Effector cells were combined with 51Cr-labeled target cells without (circles) or with 1,000 U/ml human recombinant IL-2 added for the 4-hour duration of the assay (diamonds). Results are representative of three individual experiments for patient 1 and two each for patients 2 and 3.
Induction of NK cell cytotoxicity in vivo by IL-2 in a patient with NEMO mutation.
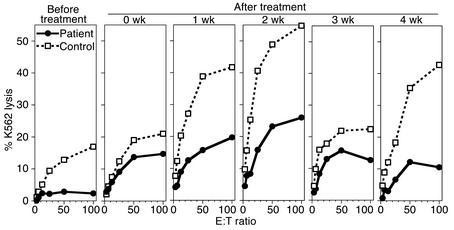
The stimulatory effect of IL-2 on in vitro NK cell cytotoxicity in NEMO patients suggested that in vivo administration of IL-2 might enhance NK activity in these patients. The recurrence of CMV disease in patient 1 despite antigen-specific antibody and lymphocyte proliferative responses, and the inability of this patient to tolerate standard anti-CMV therapy, prompted us to examine the effect of IL-2 administration on his NK activity. The patient received 1 × 106 U/m2/d rhIL-2 intravenously for 5 days; he tolerated the therapy with no adverse effects. Ex vivo NK cell cytotoxicity against K562 cells was measured prior to, immediately after, and 1, 2, 3, and 4 weeks after treatment (Figure 6). NK cell cytotoxic activity became evident immediately after therapy and persisted for at least 4 weeks. Further stimulation of patient PBMCs with IL-2 in vitro at each timepoint resulted in a fairly consistent additional increase in NK cell cytotoxicity that averaged 2.5-fold ± 0.1-fold at 50:1 E:T, suggesting that the NK cells were not maximally stimulated by in vivo treatment. These results suggest that in vivo administration of IL-2 partially overcomes the deficit in NK cell cytotoxicity in a patient with NEMO mutation.
Figure 6.
Induction of ex vivo NK cell cytotoxic activity after in vivo administration of IL-2 to a patient with NEMO exon 4 mutation. Patient 1 was treated with 1 × 106 U/m2 of human recombinant IL-2 by continuous intravenous infusion for 5 days. NK cytotoxicity was determined ex vivo by lysis of 51Cr-labeled target cells before treatment, immediately after (0 wk), 1 week after, 2 weeks after, 3 weeks after, or 4 weeks after treatment (circles). K562 lysis mediated by PBMCs from untreated control donors (squares) was performed in parallel. Different control donors were used in each assay.
Induction of NF-κB by IL-2 in a patient with NEMO mutation.
IL-2 has been demonstrated to induce activation of NF-κB in lymphocytes (28). To determine whether IL-2 was capable of activating NF-κB in lymphocytes with NEMO mutation, PBMCs from patient 1 were activated with PHA, rested, and then stimulated with IL-2. Nuclear extracts were prepared and evaluated for NF-κB binding by EMSA. NF-κB binding was increased in both patient 1 and a representative control after IL-2 stimulation (Figure 3b). Densitometric evaluation revealed a 3.7-fold increase in nuclear NF-κB binding after IL-2 stimulation for the patient and a 2.7-fold increase for the control. These data demonstrate that IL-2 is capable of inducing NF-κB in a patient with NEMO mutation and suggest the possibility that this induction may allow for IL-2–mediated enhancement of NK cell cytotoxic activity.
Discussion
We present two previously unreported mutations of human NEMO associated with HED-ID, one within the first coiled-coiled domain (L153 → R) and the other in the exon 10 zinc finger domain (Q403 → X), as well as a previously reported exon 10 mutation (C417→R) (7–9). A total of 18 NEMO mutations associated with HED-ID have been previously described. Fourteen are within exon 10, and five of these, like our patient 3, alter position 417 (7–9, 12, 13, 29). Thus, greater than 75% of NEMO mutations associated with HED-ID occur in exon 10, and 38% of these affect amino acid 417. Of the four previously described mutations outside of exon 10, only one is within the first coiled-coil domain: R175 → P (5, 8). Further immunologic studies of this mutation have not been reported.
The functional significance of exon 10 mutations has been demonstrated by the decreased ability of cells from patients to phosphorylate I-κBα and/or increase NF-κB binding activity after TNF or CD40L stimulation (8, 9). In addition, impaired B cell proliferation and cell surface upregulation of CD54 after CD40L stimulation has also been found in these patients (8, 9). The immunologic significance of the three mutations reported here is shown by impaired CD40-mediated proliferation and upregulation of B cell activation markers in all patients, and by impaired CD40-mediated IL-4–dependent IgE isotype switching in one patient (Figure 2). Further demonstration of defective NEMO signaling in our patient with the unusual exon 4 mutation was provided by diminished activation of NF-κB after ligation of CD40 (Figure 3).
The recurrent episodes of CMV with intact CMV-specific antibody response in patient 1 led us to examine NK cells. Although all patients had normal NK cell percentages and phenotype, NK cell cytotoxic activity was grossly deficient in multiple determinations in all three patients. The deficit was not due to a generalized impairment in cytotoxicity, as ADCC was robust in all three patients (Figure 4b).
Gross deficiencies of NK cell activities have been associated with increased susceptibility to herpes group virus infection in humans (15), and an individual lacking NK cells had recurrent and severe herpes group virus infections, including CMV (14). Results obtained in a murine model of CMV have demonstrated that NK cells are essential in antiviral defense (30) and that NK cell cytolytic activity is critical in viral control in certain organs (31). The ability to evaluate the contributions of NK cell cytotoxicity and ADCC to the control of CMV has not previously been possible. In particular, humans with phenotypically normal NK cells capable of mediating ADCC but lacking NK cell cytotoxicity have never been described. Mice with this phenotype have been reported, but details of their infectious susceptibility are unknown (32). A patient with a mutant CD16 molecule had phenotypically abnormal NK cells and absent NK cell cytotoxicity but normal ADCC (33). This individual had susceptibility to herpesvirus infections, further implying the importance of NK cell cytotoxicity in human control of this virus family. Our data from patients with NEMO mutation suggest that deficient NK cell cytotoxicity in the face of normal ADCC may be clinically significant.
IL-12–driven production of IFN-γ by NK cells in CMV infection is involved in murine models of infection (34). In this light, a specific inability to produce IL-12 and TNF after stimulation with CD40L or LPS has been observed in patients with NEMO mutation (8, 9). Thus it is possible that deficiency in the IL-12/IFN-γ axis may contribute to the CMV susceptibility of our patient with recurrent CMV. However, serum IFN-γ was readily detectable in this patient during symptomatic CMV disease (57 pg/ml). Therefore, the deficiency of NK cell cytotoxicity observed in patients with NEMO mutation may result in impaired defense against certain viral infections and be of clinical significance.
The involvement of NEMO in NK cell cytotoxicity is intriguing and hitherto unappreciated. NK cell cytolytic activity is believed to involve a cascade of cytoplasmic protein kinases (18). Pharmacologic inhibitors of transcription such as actinomycin D have been reported not to affect NK cell cytotoxic activity of fresh human PBMC, but to inhibit activity induced by type I IFN (35). More recent studies have shown activation of NF-κB in NK cell/target cell conjugates and that pyrrolidine dithiocarbamate inhibits activity of NF-κB but not AP-1 in cultured NK cells eliminating NK cell cytotoxic activity, suggesting a specific role for NF-κB pathways in this activity (36). If a NEMO to NF-κB pathway were to be involved in NK cell cytotoxicity, there would be several potential avenues for IKK activation. Firstly, PI3K, which is used in NK cell cytotoxicity (19, 20), activates the serine-threonine kinase Akt in NK cells (19); Akt has been reported to induce NF-κB activation (37). Secondly, proline-rich tyrosine kinase-2, which is important for NK cell cytotoxicity but not ADCC, has also been linked to NF-κB activation (21). An alternative role for NEMO in NK cell cytotoxicity, however, may be a direct, NF-κB–independent involvement of this protein in intracytoplasmic events leading to cytotoxicity. In this light, an NF-κB–independent role for IKK-α has been demonstrated in ecto-dermal development (38).
We found that IL-2 consistently induced NK cell cytotoxicity both in vitro and in vivo in patients with NEMO mutation (Figure 5 and Figure 6). This finding suggests that IL-2 may overcome the block in NK cell cytotoxicity caused by NEMO mutation. Our data raise the possibility that the IL-2–induced activity would involve NF-κB, as IL-2 was capable of inducing NF-κB in patient 1. The IL-2 signal may be able to access the mutated NEMO to allow for NF-κB activation because it may use intact domains of the mutated NEMO molecule. An alternative possibility is that IL-2 activates NF-κB and NK cell cytotoxic activity via a pathway independent of NEMO (39). It is unlikely that the IL-2 effect on NK cell cytotoxicity in patients with NEMO mutation is due to induction of TNF, as it has been reported that these patients have a grossly impaired ability to produce TNF after in vitro stimulation (8, 9).
Our findings point to an important role for NEMO and NF-κB activation in NK cell cytotoxicity. They also demonstrate that IL-2 could provide a useful therapeutic opportunity for patients with NEMO mutation because this cytokine restores deficient NK cell cytotoxicity.
Acknowledgments
We sincerely thank the patients and families affected by HED-ID that we have studied for their time, patience, and commitment to research. This work was supported by United States Public Health Service grants AI-31541 and AI-31136 (to R.S. Geha), and DE-11311 (to J. Zonana). J.S. Orange and S.R. Brodeur were supported by NIH training grant AI-07512. Clinical studies at Children’s Hospital were also supported by the Children’s Hospital General Clinical Research Center M01-RR02172 from the General Clinical Research Centers Program, National Center for Research Resources.
Footnotes
Jordan S. Orange and Scott R. Brodeur contributed equally to this work.
References
- 1.Clarke A, Phillips DI, Brown R, Harper PS. Clinical aspects of X-linked hypohidrotic ectodermal dysplasia. Arch Dis Child. 1987;62:989–996. doi: 10.1136/adc.62.10.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kere J, et al. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet. 1996;13:409–416. doi: 10.1038/ng0895-409. [DOI] [PubMed] [Google Scholar]
- 3.Monreal AW, et al. Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia. Nat Genet. 1999;22:366–369. doi: 10.1038/11937. [DOI] [PubMed] [Google Scholar]
- 4.Headon DJ, et al. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature. 2001;414:913–916. doi: 10.1038/414913a. [DOI] [PubMed] [Google Scholar]
- 5.Abinun M, Spickett G, Appleton AL, Flood T, Cant AJ. Anhidrotic ectodermal dysplasia associated with specific antibody deficiency. Eur J Pediatr. 1996;155:146–147. doi: 10.1007/BF02075774. [DOI] [PubMed] [Google Scholar]
- 6.Huntley CC, Ross RM. Anhidrotic ectodermal dysplasia with transient hypogammaglobulinemia. Cutis. 1981;28:417–419. [PubMed] [Google Scholar]
- 7.Zonana J, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am J Hum Genet. 2000;67:1555–1562. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doffinger R, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 9.Jain A, et al. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2:223–228. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 10.Li XH, Fang X, Gaynor RB. Role of IKKgamma/nemo in assembly of the IkappaB kinase complex. J Biol Chem. 2001;276:4494–4500. doi: 10.1074/jbc.M008353200. [DOI] [PubMed] [Google Scholar]
- 11.Karin M, Delhase M. The IkappaB kinase (IKK) and NF-kappaB: key elements of proinflammatory signaling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 12.Smahi A, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. Nature. 2000;405:466–472. doi: 10.1038/35013114. [DOI] [PubMed] [Google Scholar]
- 13.Aradhya S, et al. Atypical forms of incontinentia pigmenti in male individuals result from mutations of a cytosine tract in exon 10 of NEMO (IKK-gamma) Am J Hum Genet. 2001;68:765–771. doi: 10.1086/318806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 15.Fleisher G, et al. A non-x-linked syndrome with susceptibility to severe Epstein-Barr virus infections. J Pediatr. 1982;100:727–730. doi: 10.1016/s0022-3476(82)80572-6. [DOI] [PubMed] [Google Scholar]
- 16.Ballas ZK, et al. A patient with simultaneous absence of “classical” natural killer cells (CD3–, CD16+, and NKH1+) and expansion of CD3+, CD4–, CD8–, NKH1+ subset. J Allergy Clin Immunol. 1990;85:453–459. doi: 10.1016/0091-6749(90)90155-w. [DOI] [PubMed] [Google Scholar]
- 17.Moretta A, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 18.Perussia B. Signaling for cytotoxicity. Nat Immunol. 2000;1:372–374. doi: 10.1038/80808. [DOI] [PubMed] [Google Scholar]
- 19.Jiang K, et al. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- 20.Spaggiari GM, et al. NK cell-mediated lysis of autologous antigen-presenting cells is triggered by the engagement of the phosphatidylinositol 3-kinase upon ligation of the natural cytotoxicity receptors NKp30 and NKp46. Eur J Immunol. 2001;31:1656–1665. doi: 10.1002/1521-4141(200106)31:6<1656::aid-immu1656>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Gismondi A, et al. Cutting edge: functional role for proline-rich tyrosine kinase 2 in NK cell-mediated natural cytotoxicity. J Immunol. 2000;164:2272–2276. doi: 10.4049/jimmunol.164.5.2272. [DOI] [PubMed] [Google Scholar]
- 22.Sancho D, et al. The tyrosine kinase PYK-2/RAFTK regulates natural killer (NK) cell cytotoxic response, and is translocated and activated upon specific target cell recognition and killing. J Cell Biol. 2000;149:1249–1262. doi: 10.1083/jcb.149.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabara HH, et al. Role of JAK3 in CD40-mediated signaling. Blood. 1998;92:2435–2440. [PubMed] [Google Scholar]
- 24.Jabara HH, Fu SM, Geha RS, Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990;172:1861–1864. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabara HH, Brodeur SR, Geha RS. Glucocorticoids upregulate CD40 ligand expression and induce CD40L-dependent immunoglobulin isotype switching. J Clin Invest. 2001;107:371–378. doi: 10.1172/JCI10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsytsykova AV, Tsitsikov EN, Geha RS. The CD40L promoter contains nuclear factor of activated T cells-binding motifs which require AP-1 binding for activation of transcription. J Biol Chem. 1996;271:3763–3770. doi: 10.1074/jbc.271.7.3763. [DOI] [PubMed] [Google Scholar]
- 27.Lotzova E, Savary CA, Schachner JR, Huh JO, McCredie K. Generation of cytotoxic NK cells in peripheral blood and bone marrow of patients with acute myelogenous leukemia after continuous infusion with recombinant interleukin-2. Am J Hematol. 1991;37:88–99. doi: 10.1002/ajh.2830370206. [DOI] [PubMed] [Google Scholar]
- 28.Arima N, Kuziel WA, Grdina TA, Greene WC. IL-2-induced signal transduction involves the activation of nuclear NF-kappaB expression. J Immunol. 1992;149:83–91. [PubMed] [Google Scholar]
- 29.Mansour S, et al. Incontinentia pigmenti in a surviving male is accompanied by hypohidrotic ectodermal dysplasia and recurrent infection. Am J Med Genet. 2001;99:172–177. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1155>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 30.Bukowski JF, Woda BA, Welsh RM. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984;52:119–128. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tay CH, Welsh RM. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowin-Kropf B, Kunz B, Beermann F, Held W. Impaired natural killing of MHC class I-deficient targets by NK cells expressing a catalytically inactive form of SHP-1. J Immunol. 2000;165:1314–1321. doi: 10.4049/jimmunol.165.3.1314. [DOI] [PubMed] [Google Scholar]
- 33.Jawahar S, et al. Natural Killer (NK) cell deficiency associated with an epitope-deficient Fc receptor type IIIA (CD16-II) Clin Exp Immunol. 1996;103:408–413. doi: 10.1111/j.1365-2249.1996.tb08295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 35.Ortaldo JR, Phillips W, Wasserman K, Herberman RB. Effects of metabolic inhibitors on spontaneous and interferon-boosted human natural killer cell activity. J Immunol. 1980;125:1839–1844. [PubMed] [Google Scholar]
- 36.Valle Blazquez M, et al. Cellular redox status influences both cytotoxic and NF-kappaB activation in natural killer cells. Immunology. 1997;90:455–460. doi: 10.1111/j.1365-2567.1997.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozes ON, et al. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 38.Hu Y, et al. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- 39.Senftleben U, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappaB signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]