Abstract
In gastrointestinal epithelium, metaplastic conversion between predominant cell types is associated with an increased risk of neoplasia. However, the mechanisms regulating metaplastic transitions in adult epithelia are largely undefined. Here we show that matrix metalloproteinase-7 (MMP-7) is expressed not only in the majority of human pancreatic ductal adenocarcinoma specimens, but also in human pancreatic intraepithelial neoplasia and metaplastic duct lesions in human and mouse. In a mouse model of pancreatic acinar-to-ductal metaplasia, MMP-7 progressively accumulates during the metaplastic transition, resulting in a concomitant increase in solubilization of Fas ligand (FasL). Under identical conditions, mice either deficient in MMP-7 or carrying an inactive FasL gene are severely inhibited in development of progressive metaplasia and acinar cell apoptosis. Thus, MMP-7 and FasL influence the initiation and maintenance of metaplastic events in pancreatic epithelium, explaining the observed link between metaplasia and apoptosis in pancreas and other gastrointestinal tissues.
Introduction
Metaplastic conversion between epithelial cell types is frequently found at sites of chronic inflammation and confers significant risk of neoplasia to the pancreas (1–3), stomach (4, 5), intestine (6), and esophagus (7). However, little information is available regarding the molecular mechanisms responsible for initiating and maintaining metaplastic epithelia. In pancreas, acinar-to-ductal metaplasia has been proposed as an initiating mechanism for pancreatic ductal adenocarcinoma (PDAC). Patients with ductal metaplasia arising in the setting of chronic pancreatitis (CP) have a 16-fold increase in relative risk for PDAC (1), increasing to 50-fold in patients with familial CP (2). In a mouse model of pancreatic ductal metaplasia initiated by chronic overexpression of TGF-α, metaplastic ductal epithelium can progress infrequently to pancreatic intraepithelial neoplasia (PanIN) and invasive PDAC (8); this conversion from metaplasia to neoplasia is greatly accelerated on a p53–/– background (9).
Matrix metalloproteinase-7 (MMP-7, matrilysin, EC 3.4.24.23) is a member of the MMP family of zinc-dependent extracellular proteases. Unlike the majority of MMP family members, MMP-7 is expressed in the epithelium of premalignant lesions in multiple glandular tissues, including breast, prostate, and intestine (10). Although its precise function in these tumors is unknown, ablation of MMP-7 in a mouse model of intestinal neoplasia decreases tumor formation by 60% (11), and its transgenic overexpression in mammary gland accelerates the onset of Her2/Neu-induced tumors (12). These observations are consistent with a growing body of literature establishing MMPs as important regulators of tumor formation (13–15).
While many MMP substrates are components of the extracellular matrix, MMPs have recently been recognized as important regulators of cell surface proteolysis (16). Cell-surface substrates of MMP-7 include E-cadherin (17), β4 integrin (18), TNF-α (19, 20), and Fas ligand (FasL) (20). Cleavage of FasL leads to the generation of a soluble form of FasL (sFasL) that has apoptosis-inducing activity in vitro (21). While FasL has not previously been shown to be a substrate of MMP-7 in vivo, MMP7–/– mice show diminished apoptosis of prostate epithelium following androgen withdrawal (21), a process that is thought to be dependent on Fas (22). Chronic solubilization of FasL by MMP-7 in vitro leads to resistance to a number of apoptotic stimuli (23), suggesting that one role of MMP-7 in tumor formation is to select for apoptotic resistance.
Here we show MMP-7 expression in well-differentiated tumor cells in 98% of PDAC patient samples. Interestingly, we found MMP-7 in associated metaplastic duct epithelium in 100% of PDAC samples as well as in the metaplastic duct lesions of 95% of CP patient samples. In a mouse model of CP-associated acinar-to-ductal metaplasia, we found that MMP-7 was essential for propagation of metaplastic epithelium throughout the pancreatic parenchyma, and that it regulated associated acinar cell apoptosis through the solubilization of FasL. The contribution of MMP-7 activity to the formation of preneoplastic pancreatic lesions in vivo suggests that MMP-7 may influence tumor formation partly by exposing the epithelia to an apoptotic selective pressure, resulting in selective expansion of a duct-like epithelium resistant to apoptotic stimuli.
Methods
Mice, pancreatic duct ligation, and tissue harvest.
Transgenic mice expressing TGF-α under control of the metallothionein promoter (MT–TGF-α mice) (24) were maintained on a C57BL/6 × DBA genetic background. C57BL/6 male mice were used for both unoperated controls and for mice undergoing pancreatic duct ligation (PDL). MMP7–/– mice have been described previously (11); gld mice were obtained from The Jackson Laboratory (Bar Harbor, Maine, USA) and were maintained on a C57BL/6 background. PDL was performed as described previously (25).
Both unoperated control mice and mice that were 5, 7, and 10 days post-PDL were sacrificed and pancreatic tissues were harvested. Two subgroups of mice were prepared for analysis, those for immunohistochemistry and in situ hybridization and those for tissue lysates. Mice to be used for in situ hybridization and immunohistochemistry were sedated and perfused through the left ventricle of the heart with 10 ml ice-cold PBS followed by 10 ml of either 4% paraformaldehyde or formalin. For mice from which lysates were prepared, pancreatic tissue was removed and 25% of the tissue was fixed for immunohistochemistry. After harvesting, splenic lobes were fixed for 3 hours at 4°C, then washed and stored in 70% ethanol at 4°C overnight. Tissues were dehydrated and embedded in paraffin.
Protein extraction and immunoblotting.
For protein analysis, pancreatic tissues cut lengthwise from the suture point to the pancreatic tail were minced and solubilized in lysis buffer consisting of 50 mM Tris (pH 7.5), 100 mM NaCl, and 0.5% NP-40 supplemented with Complete Protease Inhibitor Tablets (Roche Molecular Biochemicals, Indianapolis, Indiana, USA). Insoluble debris was removed by centrifugation, and protein concentrations were determined using the Bradford assay (Bio-Rad Laboratories, Hercules, California, USA). Fifty micrograms of total protein per lane were loaded, resolved on SDS/12% polyacrylamide gels, and transferred to nitrocellulose membranes. Blots were blocked in 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween-20 (TBST). Antibodies and dilutions were as follows: rabbit polyclonal anti–MMP-7 antibody (11) was used at 1:200; rabbit polyclonal anti-FasL (SC-6327; Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) was used at 1:500; and rat monoclonal anti-mouse FasL antibody (Alexis Biochemicals, San Diego, California, USA) was used at 1:500. The membranes were washed in TBST and probed with biotin-conjugated species-specific secondary antibodies (The Jackson Laboratory). After washing in TBST, blots were probed with horseradish peroxidase–conjugated streptavidin and washed in TBST. Immune complexes were visualized using the ECL kit from Amersham Life Sciences Inc. (Buckinghamshire, England) and exposed to autoradiograph film. To confirm equivalent protein loading, blots were stained for total protein in a solution of 0.1% Fast Green FCF (Sigma Aldrich, St. Louis, Missouri, USA), 20% methanol, and 5% acetic acid.
Immunohistochemistry.
Archival human normal pancreas, pancreatitis, and pancreatic tumor samples that were fixed in formalin and embedded in paraffin were obtained from the Vanderbilt University Human Tissue Acquisition and Pathology Shared Resource. Five-micrometer sections from human and mouse samples were analyzed. Immunohistochemical analysis was performed as previously described (26). The following primary antibodies were used: rat monoclonal anti–MMP-7 (an antibody that recognizes both pro- and active forms of MMP-7) at 1:50 (26); rat monoclonal IgM anti–mouse FasL (Alexis Biochemicals), 1:100; rabbit polyclonal anti-Fas antibody (X-20; Santa Cruz Biotechnology Inc.), 1:200; and rabbit polyclonal antibody against cleaved caspase-3 (Cell Signaling Technology, Beverly, Massachusetts, USA), 1:100. Negative controls were prepared using appropriate species- and isotype-matched immunoglobulin. PanIN lesions were graded as either PanIN-1, PanIN-2, or PanIN-3 based on increasing degrees of epithelial stratification and nuclear atypia as previously described.
Quantitation of apoptosis and statistical analysis.
To obtain quantitative data regarding the rate of apoptosis in ligated pancreatic tissues, three fields each from five different pancreata stained with anti–cleaved caspase-3 were counted under 200× magnification. The mean and SEM were obtained, and unpaired Student t tests were performed. Statistical analysis was performed with NCSS software (Number Cruncher Statistical Systems, Kaysville, Utah, USA).
Results
MMP-7 is expressed in the majority of PDACs, PanINs, and metaplastic duct lesions.
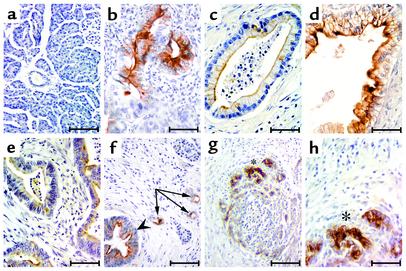
Although never detected in the normal pancreas (Figure 1a), tumor epithelium expressed MMP-7 in 31 of 32 PDAC cases examined (Figure 1, b–f). All 32 specimens contained invasive cancer. Twenty-eight of the 32 cases exhibited regions of well- to moderately well-differentiated adenocarcinoma, whereas 30 of 32 had regions of poorly differentiated adenocarcinoma. We found MMP-7 expression in well- and moderately well-differentiated invasive tumor cells in 23 of 28 specimens (Figure 1b), but never in the poorly differentiated invasive cells. Thirty-one of 32 specimens had regions containing identifiable PanIN lesions, also known as premalignant neoplasia (27). Strikingly, a subset of PanIN lesions in each positive sample expressed MMP-7. Among specimens with various stages of PanIN, we found MMP-7 in PanIN-1 lesions in 11 of 15 samples (Figure 1c), in PanIN-2 lesions in 10 of 19 samples (Figure 1d), and in PanIN-3 lesions in 14 of 19 samples (Figure 1e). Taken together, these data suggested that, in PDAC, MMP-7 has a function that precedes the onset of tumor invasion.
Figure 1.
MMP-7 expression in PDACs, PanINs, and metaplastic ducts. MMP-7 immunoreactivity in (a) normal pancreas (scale bar, 140 μm); (b) well-differentiated invasive adenocarcinoma (scale bar, 70 μm); (c) PanIN-1 (scale bar, 55 μm); (d) PanIN-2 (scale bar, 55 μm); (e) PanIn-3 (scale bar, 100 μm); (f) both PanIN-3 (arrowhead) and surrounding metaplastic duct lesions (arrows; scale bar, 100 μm); (g and h) metaplastic epithelium of CP specimen. Asterisks are used to show point of reference between g and h (scale bars represent 140 μm and 36 μm, respectively).
Consistent with a role for MMP-7 in the earliest stages of pancreatic tumorigenesis, metaplastic duct lesions within the tumor samples expressed MMP-7 in 32 of 32 PDAC cases (Figure 1f). This ductal metaplasia, which resembles that found in patients with CP, is commonly found in patients with PDAC and often is considered a reactive consequence of tumor formation. However, CP patients have recently been recognized as being at a significantly higher risk for PDAC (1, 2), suggesting that ductal metaplasia might act as a preneoplastic lesion. To address the possibility that MMP-7 expression in metaplastic duct lesions represented a host reaction to the nearby tumor, we examined MMP-7 expression in CP patients without associated neoplasia. Twenty-six of 28 CP patients were positive for MMP-7 expression, again limited exclusively to the metaplastic duct lesions (Figure 1, g and h). Thus, MMP-7 acted as a strong correlative marker for preneoplastic, metaplastic duct epithelium, independent of any influence from an associated overt tumor.
MMP-7 is expressed in mouse models of pancreatic ductal metaplasia.
Because MMP-7 has been shown to be important for tumor formation in multiple tissues (11, 12) and its expression correlates strongly with the formation of pancreatic preneoplastic lesions, we hypothesized that MMP-7 activity was important for the formation of these lesions. In order to determine whether MMP-7 played a functional role in acinar-to-ductal metaplasia, we first asked if induction of ductal metaplasia was associated with MMP-7 expression in two different mouse models. Chronic overexpression of TGF-α (8, 24, 28) has been shown to lead to acinar-to-ductal metaplasia, and infrequently to the eventual formation of PanIN-like lesions. Alternatively, obstruction of the main pancreatic duct (29, 25) accurately simulates human CP. In this model, tissue proximal to the point of obstruction exhibits all the hallmarks of CP within days, including an inflammatory response, extensive fibrosis, and acinar-to-ductal metaplasia.
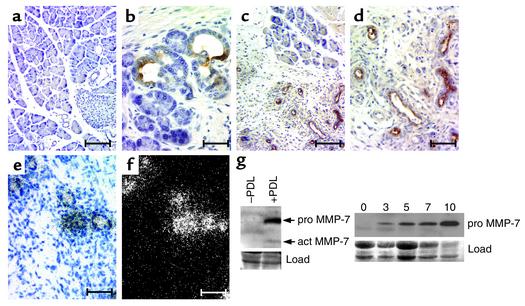
As with human pancreatic tissue, normal mouse pancreas did not express MMP-7 (Figure 2a). In MT–TGF-α mice, ductal metaplasia is extensive after 3 months of zinc-induced transgene expression (30). We observed MMP-7 expression throughout the metaplastic ductal epithelium in these mice (Figure 2b), but not in stromal or acinar tissue. Next we examined the model of CP initiated by surgical ligation of the main pancreatic duct. We examined pancreata 5 days after PDL and found MMP-7 exclusively in the metaplastic duct lesions, and not in acinar cells, fibrotic stroma, or inflammatory cells (Figure 2, c and d). In situ hybridization experiments confirmed the immunohistochemical results and showed that MMP-7 expression was induced at the RNA level (Figure 2, e and f). These data confirmed that PDL accurately simulated human CP, not only in its pathology, but also with regard to MMP-7 expression. Immunoblots of whole-tissue lysates from ligated pancreata harvested following PDL (Figure 2g) established that MMP-7 was present in both its pro and active forms. Furthermore, MMP-7 protein accumulated throughout the course of progressive metaplasia (Figure 3, a–c), consistent with its expression in the proliferative metaplastic ducts. Expression of MMP-7 in the metaplastic ducts of both MT–TGF-α and duct-ligated wild-type mice showed that, much as in human samples, MMP-7 expression is intimately linked with the process of acinar-to-ductal metaplasia, independent of the mechanism by which it is initiated.
Figure 2.
MMP-7 expression in metaplastic ducts of the mouse pancreas. MMP-7 immunoreactivity in (a) normal mouse pancreas (bar, 200 μm); (b) metaplastic ducts after chronic expression of TGF-α (scale bar, 30 μm); and (c and d) metaplastic ducts after PDL (scale bars represent 200 μm and 50 μm, respectively). (e) Bright-field and (f) dark-field views of in situ hybridization for MMP-7 in metaplastic ducts after PDL (scale bar, 100 μm). (g) First panel shows immunoblot for MMP-7 expression using lysates collected 5 days after ductal ligation. The pro (27 kDa) and active (act; 18 kDa) forms of MMP-7 are indicated. The second panel shows immunoblot for pro MMP-7 (27 kDa) using lysates from pancreata before ligation (day 0) and 3, 5, 7, and 10 days after ductal ligation, as indicated. Protein loading was visualized with Fast Green stain (Load).
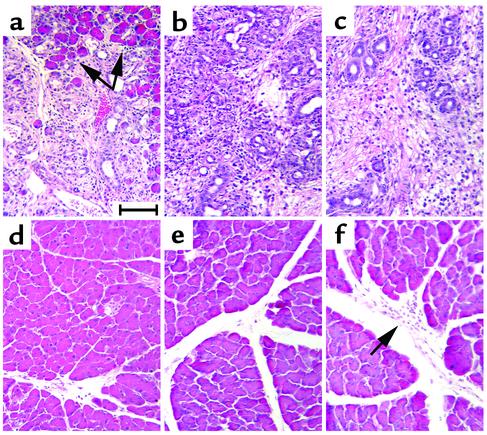
Figure 3.
Inhibition of pancreatic ductal metaplasia following duct ligation in MMP-7–/– pancreata. (a–c) Pancreatic tails of wild-type mice at (left to right) 5, 7, and 10 days after ductal ligation. Arrows in a indicate areas of persistent acinar cells. (d–f) Pancreatic tails of MMP-7–/– mice at (left to right) 5, 7, and 10 days after ductal ligation. Arrow in f indicates inflammatory cell infiltrate. Scale bar in each panel represents 100 μm.
MMP-7 controls acinar cell apoptosis and ductal metaplasia following ductal obstruction.
The consistent expression of MMP-7 during epithelial metaplasia in both human and mouse led us to hypothesize that MMP-7 played an active role in the process. Given the reliable consistency and rapidity of acinar-to-ductal metaplasia in this model, as well as its accurate simulation of human CP, we chose to study MMP-7 function in PDL-induced acinar-to-ductal metaplasia. First, we compared the histological phenotype following PDL in wild-type mice and in mice with targeted deletions of both MMP-7 alleles (MMP7–/– mice). Pancreata were harvested at 5, 7, and 10 days after PDL, and the tissue was divided into three sectors: the sector most distant from the suture point on the splenic side (tail); the suture point; and the duodenal side of the suture (head). As in previous studies (25), no changes were evident in the pancreatic head of either set of mice. In the pancreatic tail of wild-type mice, acinar cells persisted for up to 5 days after ligation (Figure 3a), but were mostly lost by 7 days after ligation (Figure 3b). In comparison, MMP7–/– mice showed few signs of either acinar cell loss or ductal metaplasia even 10 days after ligation (Figure 3, d–f), although inflammatory infiltrates were usually evident at this timepoint (Figure 3f). We found signs of CP including inflammation, fibrosis, and limited metaplasia and acinar cell loss in the MMP7–/– pancreata only immediately adjacent to the suture point, particularly at 10 days after PDL (data not shown). These observations suggest that MMP-7 plays a critical role in the propagation of metaplasia in response to ductal obstruction, but that MMP-7–independent factors were capable of inducing limited local metaplasia as a component of local foreign body reaction.
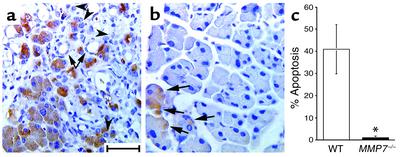
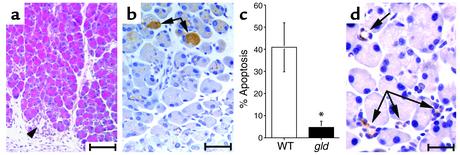
The remarkable inhibition of all histological aspects of CP in the MMP7–/– mice following PDL suggested either that the CP phenotype requires a series of MMP-7–dependent events, or (more likely) that the phenotype involves a somewhat interdependent series of events, at least one of which is initiated by MMP-7 activity. Recently, we have shown that in mice deficient for the p53 gene, acinar cell apoptosis is inhibited, which also limits the CP phenotype overall in this model (25). Given the preservation of acinar cell mass observed in the MMP7–/– mice following PDL and recent data showing that MMP-7 directly regulates epithelial cell apoptosis (21, 23, 31, 32), we hypothesized that acinar cell apoptosis was reduced relative to that found in wild-type pancreatic tissue. We examined acinar cell apoptosis by immunohistochemical staining for cleaved (active) caspase-3 of pancreata from wild-type and MMP7–/– mice 5 days after ligation. As we had seen previously (25), acinar cells in wild-type ligated pancreata exhibited extensive apoptosis, whereas metaplastic duct epithelium did not (Figure 4a). In comparison, the extent of apoptosis observed in MMP7–/– mice was similar to that in unligated controls (Figure 4, b and c).
Figure 4.
Inhibition of acinar cell apoptosis following duct ligation in MMP7–/– mice. Immunoreactivity for cleaved caspase-3 in pancreata 5 days after ductal ligation in (a) wild-type and (b) MMP7–/– mice. Arrows and arrowheads in a indicate absence of staining in metaplastic duct lesions and some acinar cells, respectively. Scale bar, 50 μm. Arrows in b indicate positively staining acinar cells. (c) Quantitation of percent apoptosis in wild-type (WT) and MMP7–/– pancreata (n = 5). *Significantly different from wild-type according to two-tailed unpaired Student t test (P = 0.007).
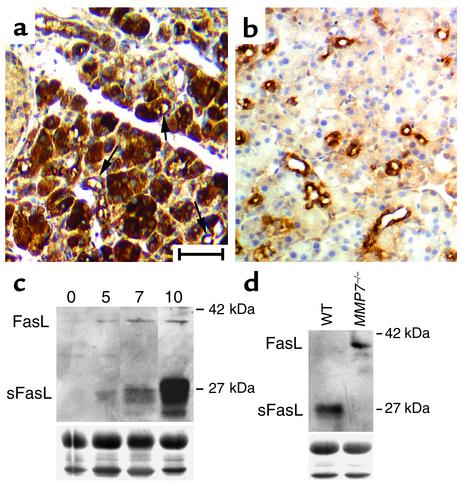
We have previously identified FasL, a member of the TNF family of apoptosis-inducing ligands, as an MMP-7 substrate in vitro (21). To investigate the possibility that MMP-7–processed FasL was relevant to initiation of ductal metaplasia, we examined the expression of FasL and its receptor, Fas, after PDL. In wild-type pancreata 5 days after PDL, we found Fas expression at very high levels in both acinar cells and metaplastic ducts (Figure 5a). In contrast, we found FasL in the exocrine pancreas restricted to metaplastic ducts (Figure 5b), a pattern similar to that found in the pancreata of CP patients (33). A subpopulation of islet cells in the endocrine pancreas expressed high levels of FasL (data not shown), a pattern observed on both sides of the suture and in unligated controls.
Figure 5.
Expression of Fas and FasL after duct ligation. Immunoreactivity for Fas (a) and FasL (b) in wild-type pancreata 5 days after ductal ligation. Bar represents 50 μm. Arrows in a indicate positive staining of metaplastic ducts among large regions of positively staining acinar cells. (c) Immunoblot of FasL in lysates isolated at 0, 5, 7, and 10 days after ductal ligation, using a rabbit polyclonal anti-FasL. Numbers indicate size markers. Full-length FasL and sFasL are indicated. (d) Immunoblot for FasL in pancreatic lysates isolated from wild-type and MMP7–/– mice 10 days after ductal ligation, using a rat monoclonal anti-mouse FasL. Protein loading in c and d was confirmed by Fast Green stain of the blot (Load).
In immunoblots of lysates from ligated pancreata, the induction of FasL expression over time was evident (Figure 5c), but strikingly, we found that the majority of FasL accumulated in its proteolytically processed, soluble form of 27 kDa, a mass consistent with the MMP-7–processed FasL. At 5 days after ligation, sFasL constituted about 60% of total FasL, increasing to more than 95% of total FasL by 10 days after ligation (Figure 5c). In MMP7–/– mice, we could detect FasL only at 10 days after ligation (presumably because of the general inhibition of metaplasia), and only in its full-length unprocessed form (Figure 5d), showing for the first time that in vivo solubilization of FasL is dependent on MMP-7 activity.
To test whether the acinar cell apoptosis and ductal metaplasia observed after PDL were dependent on FasL activity, we performed PDL on gld mice, which carry an inactivating point mutation of FasL (34). At 7 days after ligation, a time when few acinar cells persist in wild-type mice (Figure 3b), the gld mice showed virtually no acinar cell loss and an almost complete inhibition of detectable metaplastic duct formation, whereas fibrosis and inflammation were sometimes evident (Figure 6a). The persistence of acinar cells and the proapoptotic activity of FasL led us to hypothesize that acinar cell apoptosis was inhibited in gld mice. Indeed, pancreatic tissue from gld mice 7 days after PDL did show a significant decrease in acinar cell apoptosis as measured by staining of cleaved caspase-3 (Figure 6, b and c). The degree of inhibition of apoptosis was similar to that observed in the MMP7–/– background. The similarity in phenotype with the MMP7–/– mice was not due to an inhibition of MMP-7 expression, since expression of MMP-7 itself was still detectable in the gld ligated pancreata (Figure 6d). These data confirm that the apoptosis-inducing properties of MMP-7 in this system are largely dependent upon a functional FasL molecule, although the moderately higher degree of acinar cell apoptosis in the gld mice compared with the MMP7–/– mice may be an indication of alternative proapoptotic pathways controlled by MMP-7 activity. Taken as a whole, however, the progression of acinar cell apoptosis and expansion of metaplastic ductal epithelium observed in this model system was largely dependent on the function of FasL, which accumulated in the tissue primarily in its MMP-7–dependent soluble form.
Figure 6.
Inhibition of ductal metaplasia and acinar cell apoptosis in gld mice. (a) Hematoxylin and eosin staining of the pancreatic tail from a gld mouse 7 days after ductal ligation. Arrowhead indicates one of the rare metaplastic duct lesions found in these pancreata, within a region of fibrosis and inflammation. Bar, 100 μm. (b) Immunoreactivity for cleaved caspase-3 in gld pancreas 5 days after ductal ligation. Arrows indicate positively staining acinar cells. Bar, 50 μm. (c) Quantitation of percent apoptosis as indicated by staining for cleaved caspase-3, n = 5. *Significantly different from wild-type according to two-tailed unpaired Student t test (P = 0.026). (d) Immunohistochemistry for MMP-7 expression in gld pancreas 7 days after ductal ligation. Arrows indicate small intercalated ducts that stain positive for MMP-7 expression. Bar, 22 μm.
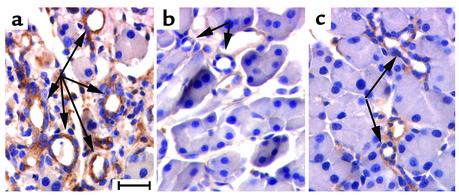
Mice deficient in MMP-7 or FasL activity are inhibited with respect to both acinar cell apoptosis and ductal metaplasia. These data, as well as our previous data showing similar results in p53-null mice (25), support the concept that acinar cell apoptosis is linked to the propagation of ductal metaplasia in this system. Interestingly, in the current study we saw that Fas receptor was expressed not only by the dying acinar cells, but also by the surviving and proliferating metaplastic duct epithelium (Figure 5). Thus, the metaplastic epithelium was protected somehow from sFasL-mediated apoptosis in order to allow for its selective expansion to become the dominant epithelium of the exocrine tissue. In a previous study we showed that metaplastic ductal epithelia express the antiapoptotic protein Bcl-2, whereas normal ducts do not (25). To test the effect of the absence of MMP-7 and FasL activity on this expression, we examined Bcl-2 by immunohistochemistry on MMP7–/– and gld pancreata following PDL (Figure 7). As shown previously, Bcl-2 is expressed specifically in the metaplastic ducts of wild-type pancreata following PDL (Figure 7a). Ductal structures in MMP7–/– pancreata did not stain for Bcl-2 (Figure 7b), consistent with the general inhibition of the metaplastic changes in this background. Surprisingly, apparently normal ducts leading to terminal acini in the gld mice did express low but detectable levels of Bcl-2 (Figure 7c). Thus, the dramatic selective expansion of ductal epithelium that is resistant to programmed cell death was dependent upon both MMP-7 activity and FasL-mediated apoptosis, with the absence of MMP-7 having a more profound effect.
Figure 7.
Bcl-2 expression in wild-type, MMP7–/–, and gld pancreata. Immunohistochemical analysis of Bcl-2 expression 7 days after ductal ligation in (a) wild-type; (b) MMP7–/–; and (c) gld pancreas. Arrows in a and c indicate metaplastic ducts that stained positive for Bcl-2. In b, arrows denote duct structures that did not stain for Bcl-2. Note that nearby acinar cells are negative for Bcl-2 staining in all samples. Bar, 36 μm.
Discussion
Here we have shown that MMP-7 is required for preneoplastic pancreatic ductal metaplasia and is expressed in pancreatic cancer precursors and well-differentiated components of invasive PDAC. We have provided evidence that following pancreatic duct obstruction, metaplastic ducts producing MMP-7 and FasL are generated. The coexpression of MMP-7 and FasL in metaplastic ducts gives MMP-7 the ability to cleave FasL from the cell surface, resulting in a local accumulation of sFasL sufficient to induce apoptosis among susceptible cell types. Despite the strong correlative and functional evidence, the profound inhibition of propagation of the CP phenotype in the MMP7–/– mice prevents us from formally eliminating the possibility that MMP-7 promotes FasL release indirectly, perhaps by enhancing inflammation and fibrosis. Nevertheless, with the induction of the apoptotic response, acinar cells are progressively eliminated and replaced by apoptosis-resistant, regenerative ductal epithelium, resulting in more cells expressing MMP-7 and FasL, continuing the cycle of acinar-to-ductal metaplasia throughout the pancreas.
Consistent with MMP-7 acting to select for cell populations resistant to apoptosis in the metaplastic process, we have observed that chronic expression of MMP-7 kills cells in vitro in a FasL-dependent manner, ultimately resulting in viable cell populations resistant to multiple apoptotic challenges, including Fas ligation, T cell–mediated killing, and chemotherapeutic agents (23). In support of this effect being due to the addition of a selective pressure, we found that chronic Fas ligation with crosslinking antibodies also leads to resistant cell populations. Similarly, in an unrelated study, chronic exposure of tissue culture cells to the apoptosis-inducing TNF-α family member TRAIL selects for resistant cell populations containing inactivating mutations in the proapoptotic protein Bax (35).
In both our in vivo and in vitro systems, it remains unclear precisely how MMP-7 activity leads to a predominance of apoptosis-resistant cells. Its effects could be indirect, caused by the application of a selective pressure that kills all but a minor, resistant cell population, or direct, caused by the release of additional signaling molecules that confer apoptotic resistance on responsive epithelia. Because Bcl-2 was detectable at low levels in ducts of the gld pancreata following PDL, it is clear that FasL-mediated apoptosis is not absolutely required for the generation of Bcl-2–expressing ductal cells, although it is clearly involved in their selective expansion. These data suggest that MMP-7 is involved in inducing Bcl-2 expression directly. However, with the slightly higher apoptotic rate in the gld mice compared with the MMP7–/– mice, we cannot eliminate the possibility that MMP-7 applies apoptotic pressure through other means, such as anoikis or release of other TNF family members. Though these remaining signals would represent a minor component of the overall apoptotic response, they may elicit sufficient selective pressure on the ductal epithelium to lead indirectly to the limited expansion of metaplastic ducts expressing Bcl-2.
Alternatively, MMP-7 may release other bioactive molecules that directly induce Bcl-2 expression and apoptotic resistance. For instance, it has been shown that MMP-7 can release heparin-binding EGF (HB-EGF) from the cell surface (32), and EGF receptor signaling is also known to induce Bcl-2 expression (36). Immunoblots examining HB-EGF in MMP7–/– pancreatic lysates did not show obvious alterations in processing compared with wild-type lysates (data not shown). Also, immunohistochemistry indicated that HB-EGF is limited to islet cells and is not found in metaplastic ducts where MMP-7 would have unfettered access to the molecule (data not shown). Whether MMP-7 activity selects for or induces Bcl-2 expression, the ultimate result is the selective expansion of ductal epithelium that is resistant to apoptotic signals that serve to eliminate the acinar cell population.
Resistance to apoptosis is a common characteristic of tumor cells and likely contributes to the ability of metaplastic ducts to act as preneoplastic lesions. The genesis of and/or selection for apoptosis-resistant epithelia would be consistent with the tumor-promoting activity of MMP-7 observed in intestine (11) and mammary gland (12). However, the ongoing high level of MMP-7 expression observed in preinvasive and well-differentiated invasive PDAC suggests that MMP-7 may also provide a growth advantage necessary for progression of pancreatic cancer precursors and perhaps initiate the early stages of PDAC invasion. Interestingly, MMP-7 positivity in PDAC lymph node metastases correlates with poor survival (37), a result consistent with any of these potential roles for MMP-7, but especially to the conference of resistance to apoptosis-inducing chemotherapy.
In vitro studies have suggested that MMP-7 expression in pancreatic cancer is a direct consequence of K-ras activation (38, 39), one of the most common genetic lesions found in pancreatic cancer (40). While K-ras is likely to influence MMP-7 expression, our own studies in pancreatic tumor cells in culture show that K-ras mutation is neither necessary nor sufficient for MMP-7 expression (Crawford et al., unpublished observations). Here we have shown that MMP-7 expression is specific to metaplastic ductal epithelium, whether induced by chronic transgenic expression of TGF-α or by induction of chronic inflammation. In the latter case at least, the short time frame from initiation to tissue harvesting combined with the high frequency of MMP-7 expression in the metaplastic ducts makes it unlikely that K-ras mutations are required for MMP-7 expression in this pancreatic ductal epithelium. Further insight into MMP-7 expression will likely come from examination of signal transduction pathways common to both inflammation and EGF family signaling. For instance, MMP-7 expression is induced by the inflammatory cytokines IL-1β and IL-6 through a Stat-3–dependent mechanism (41), and Stat-3 is downstream of EGF signaling pathways (36). Antibodies against phospho–Stat-3 did not show immunohistochemical staining specific to metaplastic ducts (data not shown), although it should be noted that phosphospecific antibodies are often problematic in tissues filled with degradative enzymes, as is the pancreas. Nevertheless, this intersection of inflammatory and EGF signaling leads to a model where ductal metaplasia in general, and MMP-7 expression specifically, are under the control of signal transduction pathways common to both methods of initiation.
The link between apoptosis, metaplasia, and neoplasia is not unique to pancreatic epithelium. Metaplasia associated with Barrett’s esophagus, ulcerative colitis, and atrophic gastritis is also associated with a dramatic increase in apoptosis of epithelial cells (42–44) and is thought in each case to act as a preneoplastic lesion (5, 6). Interestingly, each of these examples is also associated with epithelial expression of FasL (45–47) and MMP-7 (48, 49), suggesting that the observations we have made in the pancreas are likely to extend to multiple gastrointestinal epithelial tissues.
Acknowledgments
We thank David G. Johnson, Barbara Fingleton, and Anna Means for helpful discussions, and Eric Sandgren for providing TGF-α mice. This work was supported by American Cancer Society Pilot Project grant IRG-58-009-41 (to H.C. Crawford), by NIH grants R01 CA-60867 (to L.M. Matrisian), F32 CA-79107 (to C.R. Scoggins), R01 DK-56211 (to S.D. Leach), and P-1 CA-77839-01, and by Vanderbilt-Ingram Comprehensive Cancer Center Support Grant P30 CA-68485. S.D. Leach was also supported by the Paul K. Neumann Professorship in Pancreatic Cancer at Johns Hopkins University.
Footnotes
See the related Commentary beginning on page 1403.
References
- 1.Lowenfels AB, Maisonneuve P, Whitcomb DC. Risk factors for cancer in hereditary pancreatitis. International Hereditary Pancreatitis Study Group. Med Clin North Am. 2000;84:565–573. doi: 10.1016/s0025-7125(05)70240-6. [DOI] [PubMed] [Google Scholar]
- 2.Lowenfels AB, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 3.Bardeesy N, Sharpless NE, DePinho RA, Merlino G. The genetics of pancreatic adenocarcinoma: a roadmap for a mouse model. Semin Cancer Biol. 2001;11:201–218. doi: 10.1006/scbi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 4.Uemura N, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 5.Fox JG, Wang TC. Helicobacter pylori—not a good bug after all! N Engl J Med. 2001;345:829–832. doi: 10.1056/NEJM200109133451111. [DOI] [PubMed] [Google Scholar]
- 6.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jankowski JA, Harrison RF, Perry I, Balkwill F, Tselepis C. Barrett’s metaplasia. Lancet. 2000;356:2079–2085. doi: 10.1016/S0140-6736(00)03411-5. [DOI] [PubMed] [Google Scholar]
- 8.Wagner M, Luhrs H, Kloppel G, Adler G, Schmid RM. Malignant transformation of duct-like cells originating from acini in transforming growth factor transgenic mice. Gastroenterology. 1998;115:1254–1262. doi: 10.1016/s0016-5085(98)70098-8. [DOI] [PubMed] [Google Scholar]
- 9.Wagner M, et al. A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 2001;15:286–293. doi: 10.1101/gad.184701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell WC, Matrisian LM. Complex roles of matrix metalloproteinases in tumor progression. Curr Top Microbiol Immunol. 1996;213:1–21. doi: 10.1007/978-3-642-61107-0_1. [DOI] [PubMed] [Google Scholar]
- 11.Wilson CL, Heppner KJ, Labosky PA, Hogan BLM, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudolph-Owen LA, Chan R, Muller WJ, Matrisian LM. The matrix metalloproteinase matrilysin influences early-stage mammary tumorigenesis. Cancer Res. 1998;58:5500–5506. [PubMed] [Google Scholar]
- 13.Noel AC, et al. Stromelysin-3 expression promotes tumor take in nude mice. J Clin Invest. 1996;97:1924–1930. doi: 10.1172/JCI118624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sternlicht MD, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 17.Noe V, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 18.Von Bredow DC, Nagle RB, Bowden GT, Cress AE. Cleavage of beta 4 integrin by matrilysin. Exp Cell Res. 1997;236:341–345. doi: 10.1006/excr.1997.3711. [DOI] [PubMed] [Google Scholar]
- 19.Gearing AJH, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 20.Haro H, et al. Matrix metalloproteinase-7–dependent release of tumor necrosis factor-α in a model of herniated disc resorption. J Clin Invest. 2000;105:143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin (MMP-7) proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol. 1999;9:1441–1447. doi: 10.1016/s0960-9822(00)80113-x. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki A, Matsuzawa A, Iguchi T. Down regulation of Bcl-2 is the first step on Fas-mediated apoptosis of male reproductive tract. Oncogene. 1996;13:31–37. [PubMed] [Google Scholar]
- 23.Fingleton B, Vargo-Gogola T, Crawford HC, Matrisian LM. Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia. 2001;3:459–468. doi: 10.1038/sj.neo.7900190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 25.Scoggins CR, et al. p53-dependent acinar cell apoptosis triggers epithelial proliferation in duct-ligated murine pancreas. Am J Pathol. 2000;279:G827–G836. doi: 10.1152/ajpgi.2000.279.4.G827. [DOI] [PubMed] [Google Scholar]
- 26.Shattuck-Brandt RL, Lamps LW, Heppner Goss KJ, DuBois RN, Matrisian LM. Matrilysin and cyclooxygenase-2 are differentially expressed in intestinal and colorectal neoplasms. Mol Carcinog. 1999;24:177–187. [PubMed] [Google Scholar]
- 27.Hruban RH, et al. Pathology of incipient pancreatic cancer. Ann Oncol. 1999;10(Suppl. 4):9–11. [PubMed] [Google Scholar]
- 28.Jhappan C, et al. TGF-alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990;61:1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- 29.Satake K, Hiura A. A new model for pancreatitis. Pancreas. 1998;16:284–288. doi: 10.1097/00006676-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Song SY, et al. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology. 1999;117:1416–1426. doi: 10.1016/s0016-5085(99)70292-1. [DOI] [PubMed] [Google Scholar]
- 31.Mitsiades N, Yu W, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001;61:577–581. [PubMed] [Google Scholar]
- 32.Yu WH, Woessner JF, Jr, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev. 2002;16:307–323. doi: 10.1101/gad.925702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornmann M, Ishiwata T, Maruyama H, Beger HG, Korc M. Coexpression of FAS and FAS-ligand in chronic pancreatitis: correlation with apoptosis. Pancreas. 2000;20:123–128. doi: 10.1097/00006676-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 35.LeBlanc H, et al. Tumor-cell resistance to death receptor—induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 36.Zushi S, et al. STAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cells. Int J Cancer. 1998;78:326–330. doi: 10.1002/(SICI)1097-0215(19981029)78:3<326::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto H, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: clinicopathologic and prognostic significance of matrilysin expression. J Clin Oncol. 2001;19:1118–1127. doi: 10.1200/JCO.2001.19.4.1118. [DOI] [PubMed] [Google Scholar]
- 38.Ohnami S, et al. Identification of genes showing differential expression in antisense K-ras-transduced pancreatic cancer cells with suppressed tumorigenicity. Cancer Res. 1999;59:5565–5571. [PubMed] [Google Scholar]
- 39.Fukushima H, et al. Association of matrilysin mRNA expression with K-ras mutations and progression in pancreatic ductal adenocarcinomas. Carcinogenesis. 2001;22:1049–1052. doi: 10.1093/carcin/22.7.1049. [DOI] [PubMed] [Google Scholar]
- 40.Hruban RH, Iacobuzio-Donahue C, Wilentz RE, Goggins M, Kern SE. Molecular pathology of pancreatic cancer. Cancer J. 2001;7:251–258. [PubMed] [Google Scholar]
- 41.Maliner-Stratton MS, Klein RD, Udayakumar TS, Nagle RB, Bowden GT. Interleukin-1beta-induced promatrilysin expression is mediated by NFkappaB-regulated synthesis of interleukin-6 in the prostate carcinoma cell line, LNCaP. Neoplasia. 2001;3:509–520. doi: 10.1038/sj.neo.7900178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whittles CE, et al. Apoptotic and proliferative activity in the neoplastic progression of Barrett’s oesophagus: a comparative study. J Pathol. 1999;187:535–540. doi: 10.1002/(SICI)1096-9896(199904)187:5<535::AID-PATH302>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 43.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152–159. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 44.Moss SF, Calam J, Agarwal B, Wang S, Holt PR. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Younes M, Schwartz MR, Finnie D, Younes A. Overexpression of Fas ligand (FasL) during malignant transformation in the large bowel and in Barrett’s metaplasia of the esophagus. Hum Pathol. 1999;30:1309–1313. doi: 10.1016/s0046-8177(99)90061-8. [DOI] [PubMed] [Google Scholar]
- 46.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152–159. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 47.Rudi J, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Invest. 1998;102:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salmela MT, Karjalainen-Lindsberg ML, Puolakkainen P, Saarialho-Kere U. Upregulation and differential expression of matrilysin (MMP-7) and metalloelastase (MMP-12) and their inhibitors TIMP-1 and TIMP-3 in Barrett’s oesophageal adenocarcinoma. Br J Cancer. 2001;85:383–392. doi: 10.1054/bjoc.2001.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saarialho-Kere UK, et al. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol. 1996;148:519–526. [PMC free article] [PubMed] [Google Scholar]