Abstract
Dendritic cell–based (DC-based) immunotherapy represents a promising approach to the prevention and treatment of many diseases, including cancer, but current strategies have met with only limited success in clinical and preclinical studies. Previous studies have demonstrated that a TAT peptide derived from the HIV TAT protein has the ability to transduce peptides or proteins into various cells. Here, we describe the use of TAT-mediated delivery of T cell peptides into DCs to prolong antigen presentation and enhance T cell responses. While immunization of mice with DCs pulsed with an antigenic peptide derived from the human TRP2 protein generated partial protective immunity against B16 tumor, immunization with DCs loaded with a TAT-TRP2 peptide resulted in complete protective immunity, as well as significant inhibition of lung metastases in a 3-day tumor model. Although both DC/TRP2 and DC/TAT-TRP2 immunization increased the number of TRP2-specific CD8+ T cells detected by Kb/TRP2 tetramers, T cell activity elicited by DC/TAT-TRP2 was three- to tenfold higher than that induced by DC/TRP2. Furthermore, both CD4+ and CD8+ T cells were required for antitumor immunity demonstrated by experiments with antibody depletion of subsets of T cells, as well as with various knockout mice. These results suggest that a TAT-mediated antigen delivery system may have important clinical applications for cancer therapy.
Introduction
Identification of tumor antigens has provided new opportunities for the development of effective cancer therapy (1). Dendritic cell–based (DC-based) immunotherapy represents a promising approach, since DCs are potent professional antigen-presenting cells capable of initiating host immune responses against cancer and infectious and autoimmune diseases (2). Mature DCs pulsed with model antigens such as ovalbumin (OVA) and β-galactosidase (β-gal) peptides have proven effective in enhancing antitumor immunity against tumor cells expressing the same antigen (3–5). However, clinical and animal studies using mature DCs pulsed with tumor-associated self-antigens or peptides showed little success in the inhibition of tumor growth for the treatment of cancer (6–10). Although many factors could be responsible for this failure, one of the most important factors may result from the short-life of MHC class I/peptide complexes on the DC surface. Substitution of favorable key peptide residues enhances affinity of MHC/peptides or stability of the T cell receptor of a T cell specific for MHC/peptide complexes, and this enhancement has correlated with improved T cell responses and antitumor activity both in vitro and in vivo (11–13). In addition, DCs transduced with adenovirus or retrovirus encoding a tumor antigen have also enhanced antitumor immunity (14, 15).
We hypothesized that the intracellular delivery of a self-peptide into mature DCs by a cell-penetrating peptide (CPP) may allow DCs to process and present the internalized peptides to T cells by newly synthesized MHC class I molecules for an extended time. Several CPPs have been identified from proteins, including the Tat protein of HIV (16), the VP22 protein of herpes simplex virus (17, 18), and FGF (19, 20). Among them, the 11-mer TAT peptide (YGRKKRRQRRR) has been well studied for the transduction of biologically active proteins into cells both in vitro and in vivo (21–24). However, the effectiveness of antitumor immunity elicited by DCs loaded with TAT-self-peptide has not been demonstrated in animal tumor models. Since the majority of tumor antigens are self-antigens (1, 25), evaluation of DCs loaded with TAT-TRP2 should provide critical information for its application for the treatment of cancer.
In this study, we describe the use of TAT peptide (YGRKKRRQRRR) covalently fused to a TRP2 peptide (SVYDFFVWL) for intracellular delivery to allow DCs to continuously present MHC/peptide to T cells for an extended time. We show that immunization of DCs loaded with the TAT-TRP2 peptide resulted in complete protection of mice from subsequent B16 tumor challenge, as well as in significant inhibition of lung metastases in a 3-day tumor model. Both CD4+ and CD8+ T cells were required for generating antitumor immunity using either antibody depletion of a subset of T cells or various knockout (KO) mice. These studies indicate that TAT-mediated antigen delivery into DCs could significantly enhance antitumor immune responses. Thus, this approach may improve the clinical outcome of DC-based cancer therapy.
Methods
Cell lines.
B16 is a pigmented mouse melanoma cell line of C57BL/6 (B6) origin. EL4 is a lymphoma cell line. These cell lines were maintained at 37°C and 5% CO2 in RPMI 1640 supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (Biofluids Inc., Rockville, Maryland, USA), and 2.5 mg/ml of Fungizone (Life Technologies Inc., Gaithersburg, Maryland, USA). I-Ab cells were established by transfecting plasmid DNAs encoding murine I-Ab (α and β chains) into HEK293 cells. I-Ab–positive 293 cells were cloned by a limiting dilution method, and screened by anti–I-Ab antibody.
Peptides.
The TRP2 peptide used in this study is a nine–amino acid sequence (SYVDFFVWL), derived from the TRP2 protein (26, 27). Control H2-Kb–restricted peptide was β-gal (DAPIYTNV). The TAT peptide used in this study is an 11-mer (YGRKKRRQRRR). TAT-TRP2 (YGRKKRRQRSRRYVDFFVWL), TAT-ESO (YGRKKRRQRRRASGPGGGAPR), TAT–β-gal (YGRKKRRQRRRDAPIYTNV), ES-TRP2 (MRYMILGLLALAAVCSASYVDFFVWL), and ES-OVA (MRYMILGLLALAAVCSASIINFEKL) peptides were synthesized and purified by HPLC. All peptides were dissolved in DMSO, and diluted in PBS for final concentrations.
DC preparation.
Mouse DCs were derived from B6 bone marrow using murine IL-4 and GM-CSF, as described previously (28). Briefly, bone marrow was obtained from tibia and femurs by flushing them with media. After lysis of red blood cells, cells were resuspended at 106 cells in RPMI 1640 supplemented with 5% FBS, L-glutamine (2 mM), penicillin/streptomycin (50 U/ml), 10% nonessential amino acids, and 50 mM 2-mercaptoethanol plus 1000 U/ml of recombinant GM-CSF and 1000 U/ml of IL-4 (Peprotech Inc., Rocky Hill, New Jersey, USA). Five milliliters of cells were then plated per well in six-well plates and incubated at 37°C, 5% CO2. Fresh medium supplemented with GM-CSF and IL-4 was added on days 2 and 4, and all loosely adherent cells on day 6 were transferred to 10-cm Petri dishes. Nonadherent cells were harvested and pulsed for 1 hour at 37°C with peptides in Opti-MEM media (GIBCO BRL; Life Technologies Inc.), washed three times with PBS, and used for mouse injections (3 × 105 cells intravenously per mouse).
Flow cytometry analysis.
DCs were stained with either fluorescein (FITC)-conjugated antibodies against murine I-Ab, CD80, and CD86 or R-phycoerythrin–conjugated (R-PE–conjugated) antibodies against CD3, NK, and B220, respectively. For double staining, DCs were stained with FITC–conjugated anti–I-A, anti-CD80, or anti-CD86 antibodies, and then stained with PE–conjugated anti-CD11c antibody. FITC–conjugated and PE–conjugated isotype control antibodies were used to stain DCs as a control. Double staining of DCs with anti-NK or anti-B220 followed by anti-CD3 antibodies was used to determine the contamination of DCs with NK, T, and B cells. Cells were analyzed on a flow cytometer (FACScan; Becton Dickinson Immunocytometry Systems, San Jose, California, USA).
Tetramer analysis.
Kb/TRP2 tetramers were obtained from Beckman Coulter Inc. (Miami, Florida, USA). Single suspended cells were prepared from the spleens of the immunized mice, and stained with anti-CD8–FITC antibody. After three washes with PBS, 10 μl of Kb/TRP2 tetramer was added to the tube containing 100 μl of freshly isolated T cells, mixed gently, and incubated for 30 minutes at room temperature. After three washes with PBS, T cells were resuspended in 500 ml of PBS/0.5% PFA and were ready for FACS analysis.
Mice and tumor treatment.
Six- to eight-week-old female B6, CD4 KO, CD8 KO, and class I and II KO mice were purchased from the National Cancer Institute (NIH), Taconic (Germantown, New York, USA), or The Jackson Laboratory (Bar Harbor, Maine, USA), and maintained in a pathogen-free mouse facility at Baylor College of Medicine. The CD4 KO and CD8 KO mice were obtained after backcross to B6 mice for more than ten generations. For the 3-day tumor model, all mice were injected intravenously through the tail vein with 3 × 105 B16 melanoma cells. After 3 days, mice were injected intravenously with 3 × 105 peptide-loaded DCs. Fourteen days later, lungs were removed and metastases enumerated in a blinded fashion. For the prevention model, tumor challenge was performed 14 days after immunization with DC/peptides. Two weeks later, mice were sacrificed, all lobes of both lungs were dissected, and metastases were counted. For antibody depletion, 200 μg of anti-CD4 (GK1.5), anti-CD8 (2.43), or control antibodies in 500 μl of PBS were injected intraperitoneally into each mouse on the day before tumor challenge, followed by three injections on days 1, 3, and 10 after tumor injection. Depletion of CD4+ or CD8+ T cells was determined by FACS analysis.
T cell stimulation with peptides in vitro and T cell activity assays.
2 × 106 splenocytes freshly prepared from the immunized mice (two per group) were grown in 24-well plates with RPMI 1640/5% mouse serum (without IL-2). Peptides were added to wells to a final concentration of 10 μM. T cell cultures were incubated for 6 days. For T cell assay, EL4 and 293I-Ab cells were pulsed with 10 μM of each peptide for 2 hours at 37°C. Following three washes with RPMI 1640 containing 5% mouse serum and glutamine, 1 × 105 target cells were cocultured with T cells (2 × 105) in RPMI containing 5% mouse serum, glutamine, and IL-2 (120 IU/ml) for 18 hours at 37°C and 5% CO2. All cell culture supernatants were harvested after overnight incubation and stored at –20°C until use. Murine IFN-γ or GM-CSF release was determined with ELISA kits (Endogen Inc., Woburn, Massachusetts, USA) according to the manufacturer’s instructions.
Results
TAT-mediated intracellular delivery of TRP2 into DCs for eliciting protective immunity.
Both human and murine TRP2 proteins were recently identified as tumor antigens recognized by CD8+ T cells (26, 29). Of particular interest, human HLA-A2–restricted TRP2 peptide (SVYDFFVWL) is identical to murine peptide presented by Kb molecules (27), representing an ideal human tumor antigen to be tested in animal models. In addition, combination therapy of B16–GM-CSF tumor vaccine with cytotoxic T lymphocyte antigen–4 blockade revealed that CD8+ T cells from the treated mice recognized the TRP2 peptide, but did not respond to other tumor antigens such as TRP1/gp75, tyrosinase, or MART-1 (30), suggesting that TRP2 is a dominant tumor rejection antigen. However, several attempts using TRP2 peptide, DNA, or adenovirus containing murine TRP2 failed to demonstrate the effective induction of protective or therapeutic immunity against B16 tumor (31–33). Multiple immunization with DCs pulsed with TRP2 or DNA immunization with a xenogeneic form of TRP2 (human TRP2) generated partial protective immunity (9, 33). It appeared that cytotoxic T lymphocytes (CTLs) detected in vitro after vaccination were not sufficient to inhibit tumor growth (31). We have obtained similar results using DCs pulsed with the TRP2 peptide to immunize mice (data not shown). The limited capacity of DC/peptide vaccination to induce effective T cell responses may be due to the relatively short half-life of surface MHC/peptide complexes (34). We reasoned that intracellular delivery of the TRP2 peptide into DCs by a peptide (YGRKKRRQRRR) from HIV Tat protein, which has been shown to efficiently deliver both peptides and proteins into cells in vitro and in vivo (21–24), would provide continuous supplies of peptide to be processed and presented by newly synthesized MHC class I molecules on the DC surface for T cell activation, thus leading to potent antitumor immunity. Incubation of DCs with the TAT-TRP2–FITC peptide resulted in transduction of 100% of cells (data not shown), which was in agreement with previous findings showing that a TAT–β-gal fusion protein was capable of efficiently delivering peptides or proteins into different types of cells (21, 22).
To test whether mature DCs loaded with the TAT-TRP2 peptide could generate potent protective immunity against B16 tumor, we prepared mature DCs from bone marrow of B6 mice in the presence of GM-CSF and IL-4. The phenotype of MHC class II and costimulatory molecules of mature DCs is shown in Figure 1a. After incubation of DCs with TRP2, TAT-TRP2, TAT-ESO, ES-TRP2, and ES-OVA peptides, B6 mice were immunized by intravenous injection of 3 × 105 mature DC/peptides, and boosted with DC/peptide once 2 weeks after the first vaccination. The endoplasmic reticulum insertion signal sequence (ES) was reported to enhance T cell responses when fused to OVA as a minigene or to MART-1 peptide (35, 36). For direct comparison, ES-TRP2 was included in our experiments, while TAT-ESO and ES-OVA were used as control peptides. Two weeks after the last immunization, mice were challenged with 3 × 105 B16 tumor cells. Lungs of mice were harvested and lung pulmonary metastases counted after 2 weeks of tumor challenge. Mice immunized with DC/TAT-TRP2 were completely protected from tumor challenge (P < 0.0007), while mice immunized with DC alone or DC/control peptides developed lung metastases (Figure 1b). Immunization with ES-TRP2 produced partial protection of mice from B16 tumor challenge at a level similar to that of mice that received DC/TRP2 vaccine (P < 0.05), suggesting that the ES sequence did not enhance antitumor immunity. Subsequent experiments showed that a single injection of DC/TAT-TRP2 was sufficient to generate complete protective immunity against B16 tumor, whereas other treatments were ineffective in protecting mice from tumor challenge (data not shown).
Figure 1.
Intracellular delivery of TRP2 peptide into DCs for the generation of potent antitumor immunity. (a) Surface phenotype of DCs generated from bone marrow of B6 mice. DCs were stained with FITC–conjugated anti–I-A, anti-CD80, anti-CD86, or other antibodies, followed by staining with PE–conjugated anti-CD11c, and analyzed by flow cytometry. The frequency of double positive staining for CD11c and I-A, CD80, or CD86 was over 65%, while staining for CD3, B220, and NK1.1 was negative. (b) Immunization of mice with mature DCs pulsed with TAT-TRP2 generates protective immunity. B6 mice were immunized with DCs loaded with each indicated peptide; this was followed two weeks later by intravenous injection of B16 tumor cells. The number of lung metastases was counted after 14 days of B16 tumor challenge. Mean numbers (n = 8 per group) of lung metastases ± SEM are presented. Statistical significance of differences between the control group and testing groups: *P < 0.05; **P < 0.0007. Spl, splenocytes. (c) Mice were immunized with DCs loaded with gp70 (control peptide), TRP2, and TAT-TRP2 peptides twice and were then challenged with B16 tumor cells. Animal survival was monitored up to 60 days after tumor challenge. Two independent experiments (n = 5 per group in experiment 1 and n = 10 per group in experiment 2) are shown. The survival advantage of the groups receiving DC/TAT-TRP2 treatment over the control groups receiving DC/gp70 or TAT–β-gal is statistically significant (P < 0.001), as determined by the log rank test.
To test whether DC/TAT-TRP2 immunization could prolong the survival of mice after B16 tumor challenge, we immunized mice with DC/control peptides (gp70 or TAT–β-gal), DC/TPR2, and DC/TAT-TRP2, twice. Two weeks after the last immunization, mice were challenged with B16, and the surviving mice were monitored for 60 days. As shown in Figure 1c, the survival rate in mice immunized with DC/TAT-TRP2 was significantly improved in two independent experiments as compared with that in mice receiving DC/control peptide or DC/TRP2 peptides (P < 0.001). Although DC/TRP2 slightly prolonged survival, all mice died before day 54. By contrast, 30–60% of the mice that received DC/TAT-TRP2 vaccine remained alive at day 60. Taken together, these results clearly indicate that TAT-mediated intracellular peptide delivery considerably enhances protective immunity against B16 tumor.
Therapeutic immunity against B16 tumor.
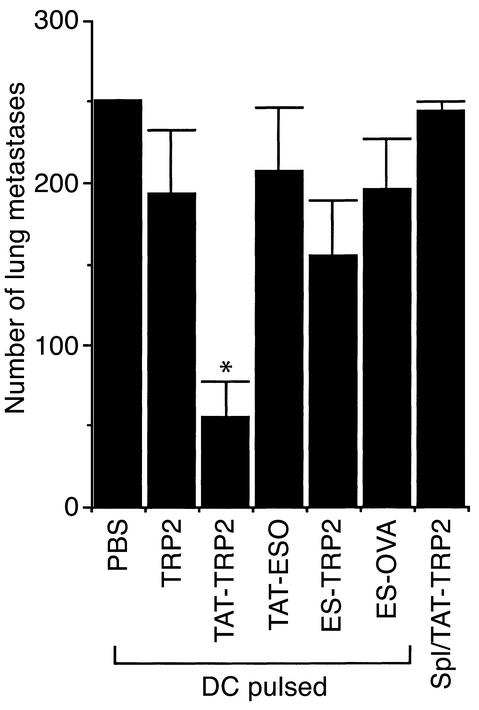
We next tested whether immunization with DCs loaded with TAT-TRP2 could generate immune responses strong enough to inhibit B16 tumor in a 3-day tumor model. Three days after intravenous injection of B16 tumor cells (3 × 105 cells per mouse), the animals were immunized with a single intravenous injection of DCs loaded with various peptides. Lungs were harvested and lung metastases were counted on day 18. Mice immunized with DC/TRP2, TAT-ESO, ES-TRP2, and ES-OVA peptides failed to reduce the number of lung metastases compared with the control group immunized with DC/PBS (Figure 2). Immunization of mice with DC/TAT-TRP2, however, significantly reduced the number of lung metastases (P < 0.05). Immunization with spleen cells pulsed with TAT-TRP2 did not show any inhibitory effect on tumor growth, indicating that effective immunization requires mature DCs as well as TAT-TRP2. DCs pulsed with the 11-mer TAT peptide plus the TRP2 peptide or with irrelevant peptide–TRP2 did not elicit any antitumor immunity against B16 tumor (data not shown).
Figure 2.
Therapeutic effect on B16 tumor growth after immunization with peptide-loaded DCs. Inhibition of tumor growth by DC/TAT-TRP2 immunization is shown. B6 mice were intravenously injected with B16 tumor cells. After 3 days, mice were immunized with DCs loaded with various peptides. Fourteen days after vaccination, lung metastases were counted in a blinded fashion. Mean numbers of lung metastases are presented. *Significant difference between TAT-TRP2 and other groups (P < 0.05), as determined by the t test. Splenocytes pulsed with DCs/TAT-TRP2 did not reduce the number of lung metastases.
TRP2-specific CD8+ T cells confer protective immunity.
To test whether T cell activity was correlated with enhanced antitumor immunity, we immunized B6 mice with DCs/peptides and evaluated T cell response from the splenocytes of the immunized mice. Previous studies showed that at least two or three immunizations with DC/TRP2 were required for inducing protective immunity against B16 tumor cells (9, 37), while one immunization with DC/TAT-TRP2 was sufficient for providing the protection of mice from tumor challenge. Thus, we evaluated T cell responses after one as well as two immunizations. Splenocytes were harvested from the immunized mice and stimulated in vitro with the same peptide used for vaccination for 6 days. We found that after one immunization, TRP2-specific T cell activity was readily detected in the mice immunized with DC/TAT-TRP2, but not in those immunized with DC/TRP2 (Figure 3a). EL4 cells pulsed with TAT–β-gal were used as a control for the specificity of T cell recognition.
Figure 3.
Induction of CD8+ T cell responses after vaccination. (a) Recognition of target cells by T cells generated from splenocytes of mice immunized once with DCs pulsed with the TRP2 or TAT-TRP2 peptides. Untreated mice were used as controls. (b) T cells were generated from splenocytes of mice after two immunizations. The splenocytes of two mice from each group were restimulated with the same immunized peptides in vitro, and T cells were tested against EL4 or 293I-Ab cells pulsed with TRP2, TAT-TRP2, or TAT–β-gal peptides, as well as against B16 tumor or MHC class I–matched EL4 tumor cells. T cell activity was determined on the basis of IFN-γ release measured by ELISA.
After two immunizations, TRP2-specific as well as B16 tumor–reactive T cells were detected in the mice immunized with either DC/TRP2 or DC/TAT-TRP2. However, T cell recognition for EL4/TRP2 and B16 tumor cells in the DC/TAT-TRP2 group was at least ten- and threefold better than that in the DC/TRP2 vaccine group, respectively. No T cell activity was detected against MHC class I–matched EL4 cells alone, or pulsed with TAT–β-gal peptide. In addition, although T cells from the DC/TAT-TRP2–immunized mice could not respond to 293I-Ab cells expressing murine MHC class II molecule (murine class I Kb molecule–negative), or 293I-Ab cells pulsed with TRP2 or TAT–β-gal peptides, they were capable of recognizing 293I-Ab cells pulsed with the TAT-TRP2 peptide (Figure 3b), suggesting the existence of I-Ab–restricted CD4+ T cell activity. CD4+ T cell activity was retained even after the depletion of CD8+ T cells (data not shown). This may explain why T cell activity against EL4 cells pulsed with the TAT-TRP2 peptide was higher than that against EL4 pulsed with the TRP2 peptide (Figure 3b). No T cell activity was detected against EL4/TAT-TRP2 target cells from the mice immunized with DC/TRP2 (data not shown).
To further evaluate TRP2-specific T cells in fresh splenocytes from the immunized mice, we prepared suspensions of single spleen cells and stained them with anti-CD8+ FITC-conjugated antibody and PE conjugated Kb/TRP2 tetramers. As shown in Figure 4, while the background staining for CD8+ and Kb/TRP2 tetramers in untreated mice was only 0.46%, results for double positive staining of TRP2-specific CD8+ T cells in DC/TRP2 and DC/TAT-TRP2 vaccination groups were 3.65% and 2.49%, respectively. Thus, these T cells appear responsible for the T cell activity detected in vitro, although the percentage of such TRP2-specific T cells in the DC/TRP2 mice was not correlated with T cell activity detected in vitro (Figure 3b), suggesting a possible functional defect or unresponsiveness of T cells from the mice immunized with DC/TRP2. This may account, in part if not entirely, for the weak immunity against B16 tumor cells.
Figure 4.
Characterization of TRP2-specific CD8+ T cells in spleens. Splenocytes were stained with anti-CD8 FITC-conjugated antibody and Kb/TRP2 tetramers. Naive mice were used as controls for the background staining. Numbers in the upper right quadrants represent the percentage of TRP2-specific CD8+ T cells in total splenocytes.
CD4+ T cells are required for the enhanced antitumor immunity.
It has been reported that a lack of CD4+ T cell response may lead to weak CD8+ T cell responses or even unresponsiveness against tumor cells (38–41). To test whether CD4+ T cells are required for protective immunity in vivo, CD4+ or CD8+ T cells were depleted in mice previously immunized with DC/TAT-TRP2 peptide with specific antibodies against CD4 or CD8 molecules. Anti-CD4, anti-CD8, or control antibodies were injected intraperitoneally on the day before B16 tumor challenge, followed by three injections on days 1, 3, and 10 after tumor injection. Mice immunized with DC/TAT-TRP2 rejected tumor growth after tumor challenge. After treatment with the control antibody, they retained the ability to inhibit tumor growth. However, the groups of mice receiving either anti-CD8 or anti-CD4 antibody showed a strikingly diminished protection from tumor challenge (Figure 5a), resulting in an increase in the number of lung metastases.
Figure 5.
CD4+ and CD8+ T cells are required for antitumor immunity. (a) B6 mice were immunized with DC/TAT-TRP2. Mice were treated with anti-CD4, anti-CD8, and control antibodies on the day before tumor challenge, followed by three injections on days 1, 3, and 10 after tumor injection. Mice immunized with PBS, DC/PBS, and DC/TAT–β-gal were used as control groups for tumor injection and specificity. Lung metastases were counted in each group (n = 5). Similar results were obtained in three repeated experiments. (b) B6, CD4 KO, and CD8 KO mice were immunized with DC/TAT-TRP2, and then challenged with B16 tumor cells. DC/PBS was used as a control (n = 5 per group). The number of lung metastases was counted after 14 days of tumor challenge and plotted as mean numbers of lung metastases. (c) DCs were prepared from class I KO, class II KO, and B6 mice, pulsed with the TAT-TRP2 peptide, and used for immunization of B6 mice. DC/PBS was used as a negative control group. Nonimmunized KO mice failed to produce protective immunity against tumor challenge. The experimental procedure for tumor challenge was the same as shown above.
To further confirm the role of CD4+ T cells in antitumor immunity, we also immunized wild-type, CD4 KO, and CD8 KO mice with TAT-TRP2–loaded DCs. DCs used for immunization were generated from wild-type B6 mice and expressed both class I and class II molecules. The wild-type B6 mice immunized with DC/PBS and DC/TAT-TRP2 were used as negative and positive controls, respectively. As expected, B6 mice immunized with DC/PBS failed to reject tumor challenge, while those vaccinated with DC/TAT-TRP2 showed completely inhibited tumor growth (Figure 5b). By contrast, CD4 KO and CD8 KO mice receiving the DC/TAT-TRP2 vaccine could not eliminate B16 tumor, resulting in an increase in the number of lung metastases in both groups. These results suggest that both CD4+ and CD8+ T cells are required for antitumor immunity.
We also generated DCs from MHC class I KO, class II KO, and B6 (wild-type) mice and pulsed them with the TAT-TRP2 peptide. DCs derived from MHC class I KO mice could present MHC class II/peptide, but not class I/peptide complexes on the cell surface, while DCs from class II KO mice could present class I/peptide complexes only. B6 mice were immunized with class I KO–derived DC/TAT-TRP2, class II KO–derived DC/TAT-TRP2, or B6-derived DC/TAT-TRP2. Two weeks later, the immunized mice were challenged with B16 tumor cells. As shown in Figure 5c, neither MHC class I nor class II KO DC/TAT-TRP2 could reject B16 tumor. By contrast, immunization with B6 DC/TAT-TRP2 resulted in tumor rejection. These results further suggest that stimulation of either CD4+ or CD8+ subsets of T cells is insufficient to eliminate B16 tumor.
Discussion
Presentation of self-antigens on the DC surface may be limited by the rapid turnover of MHC class I molecules or the binding affinity of peptides to MHC class I molecules (42). Thus, prolonged presentation of MHC/peptide complexes appears to be essential for the induction of effective antitumor immunity. In this study, we demonstrated that TAT-mediated peptide delivery can enhance the efficacy of cancer immunotherapy. Although the mechanism of peptide penetration across a lipid bilayer is not clear, the TAT peptide or its fusion proteins can rapidly and efficiently enter into cells at 4°C and are independent of receptor- or endosome-mediated endocytosis (43). Interestingly, TAT-mediated delivery somehow works more efficiently in the form of peptides or fusion proteins than in a DNA fusion construct, while VP22 is significantly less effective when used in the form of purified fusion proteins compared with VP22 DNA fusion constructs (43, 44). Because TAT-tumor peptides can be chemically made in large quantities and the amino acid sequences of many T cell peptides, including MHC class I– and class II– restricted peptides, are known, delivery of these peptides into DCs offers a new way to enhance antitumor immune responses. A previous report showed that immunization with DC/OVA protein conjugated with a nine–amino acid TAT peptide enhanced CTL responses at a level comparable to that achieved with DC/OVA peptide, but in vivo antitumor activity was not assessed in the study (45). We report here that DC/TAT-TRP2 immunization generates potent antitumor immunity against poorly immunogenic B16 tumor cells in an animal model. Although TAT fusion proteins may provide a superior antigen source because they contain multiple T cell epitopes, their use is limited by the need to obtain a large amount of the purified fusion proteins. Any residual contamination by bacterial proteins may cause undesirable immune responses. Possibly, the TAT peptide may affect the maturation of DCs. To evaluate this possibility, we incubated immature DCs with TRP2 or TAT-TRP2 for 2 hours and then analyzed expression levels of MHC class I, MHC class II, B7.1, B7.2, CD11c, and CD40 by FACS. No significant difference in expression level for maturation markers was found after TRP2 and TAT-TRP2 incubations (data not shown).
Previous studies showed a discrepancy between T cell activity detected in vitro and antitumor activity in vivo in animal models (9, 31, 33, 37) as well as in cancer patients (7, 12, 46). We attempted to enhance our ability to analyze T cell responses by class I/peptide tetramer staining and in vitro stimulation of T cells with murine serum–containing (instead of FCS-containing) medium. We found that although 2.5–3.5% of TRP2-specific CD8+ T cells were detected in DC/TRP2 and DC/TAT-TRP2 vaccination groups, CD8+ T cells in the DC/TRP2 group failed to produce a large amount of IFN-γ after exposure to TRP2 peptide or B16 tumor cells compared with T cells induced by DC/TAT-TRP2 vaccination, suggesting that these T cells exhibit functional defect or unresponsiveness (Figures 3 and 4). Preliminary analysis of lymph nodes did not reveal a significant difference between groups receiving DC/TRP2 and DC/TAT-TRP2 (data not shown). Potent T cell activity elicited by DC/TAT-TRP2 appears responsible for antitumor activity in vivo. Similarly, although antigen-specific T cells increased as determined by tetramer staining in cancer patients after peptide vaccination, these tyrosinase-specific T cells show little or no cytolytic activity against peptide-pulsed target cells or tumor cells and fail to produce cytokines (47).
The functional defects of T cells in the DC/TRP2 group may result from a lack of CD4+ T cell response. Interestingly, a weak CD4+ T cell activity was detected in the mice vaccinated with DC/TAT-TRP2 (Figure 3), suggesting that CD4+ T cells specific for 293I-Ab/TAT-TRP2 might play a role in preventing the unresponsiveness of TRP2-specific CD8+ T cells. Data from CD4 KO and CD8 KO mice as well as mice with antibody depletion of subsets of T cells further support the notion that CD4+ T cells are critical for potent antitumor immunity (Figure 5). More importantly, stimulation of either CD4+ or CD8+ T cells alone by class I KO–derived or class II KO–derived DCs failed to reject B16 tumor (Figure 5c). Previous studies also showed that CD4+ T cells are required for an antitumor effect in vivo elicited by both TRP1 and TRP2 DNA or by protein vaccination (25, 32, 33). Understanding of the mechanism by which CD4+ T cells play a role in antitumor immunity is critical for developing more effective vaccines against cancer and requires further investigation (48). Finally, it was reported that carryover T helper epitopes from FBS may enhance T cell response (49). In addition, both tumor-specific and -nonspecific (unrelated) T helper peptides could provide critical help for eliciting CTL immunity (50). We are currently investigating whether T cell responses could be further augmented by incorporating tumor-related MHC class II peptide from tumor antigens such as NY-ESO-1 and class I–restricted TRP2 peptide into vaccine regimens.
Acknowledgments
We wish to thank Steven A. Rosenberg for his generous support and encouragement. This work is supported in part by the funds of Baylor College of Medicine and by a grant from the NIH (R01-CA90327-01A1).
Footnotes
Helen Y. Wang and Tihui Fu contributed equally to this work.
References
- 1.Wang RF, Rosenberg SA. Human tumor antigens for cancer vaccine development. Immunol Rev. 1999;170:85–100. doi: 10.1111/j.1600-065x.1999.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young JW, Inaba K. Dendritic cells as adjuvants for class I major histocompatibility complex-restricted antitumor immunity. J Exp Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nestle FO, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 7.Thurner B, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellone M, et al. Relevance of the tumor antigen in the validation of three vaccination strategies for melanoma. J Immunol. 2000;165:2651–2656. doi: 10.4049/jimmunol.165.5.2651. [DOI] [PubMed] [Google Scholar]
- 9.Schreurs MW, et al. Dendritic cells break tolerance and induce protective immunity against a melanocyte differentiation antigen in an autologous melanoma model. Cancer Res. 2000;60:6995–7001. [PubMed] [Google Scholar]
- 10.Dallal RM, Lotze MT. The dendritic cell and human cancer vaccines. Curr Opin Immunol. 2000;12:583–588. doi: 10.1016/s0952-7915(00)00146-1. [DOI] [PubMed] [Google Scholar]
- 11.Parkhurst MR, et al. Improved induction of melanoma reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A0201 binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 12.Rosenberg SA, et al. Immunologic and therapeutic evaluation of a synthetic tumor-associated peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slansky JE, et al. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000;13:529–538. doi: 10.1016/s1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 14.Reeves ME, Royal RE, Lam JS, Rosenberg SA, Hwu P. Retroviral transduction of human dendritic cells with a tumor-associated antigen gene. Cancer Res. 1996;56:5672–5677. [PubMed] [Google Scholar]
- 15.Kaplan JM, et al. Induction of antitumor immunity with dendritic cells transduced with adenovirus vector-encoding endogenous tumor-associated antigens. J Immunol. 1999;163:699–707. [PubMed] [Google Scholar]
- 16.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 17.Elliott G, O’Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 18.Phelan A, Elliott G, O’Hare P. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat Biotechnol. 1998;16:440–443. doi: 10.1038/nbt0598-440. [DOI] [PubMed] [Google Scholar]
- 19.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 20.Rojas M, Donahue JP, Tan Z, Lin YZ. Genetic engineering of proteins with cell membrane permeability. Nat Biotechnol. 1998;16:370–375. doi: 10.1038/nbt0498-370. [DOI] [PubMed] [Google Scholar]
- 21.Fawell S, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 23.Lindgren M, Hallbrink M, Prochiantz A, Langel U. Cell-penetrating peptides. Trends Pharmacol Sci. 2000;21:99–103. doi: 10.1016/s0165-6147(00)01447-4. [DOI] [PubMed] [Google Scholar]
- 24.Caron NJ, et al. Intracellular delivery of a Tat-eGFP fusion protein into muscle cells. Mol Ther. 2001;3:310–318. doi: 10.1006/mthe.2001.0279. [DOI] [PubMed] [Google Scholar]
- 25.Houghton AN, Gold JS, Blachere NE. Immunity against cancer: lessons learned from melanoma. Curr Opin Immunol. 2001;13:134–140. doi: 10.1016/s0952-7915(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 26.Bloom MB, et al. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–460. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parkhurst MR, et al. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2) Cancer Res. 1998;58:4895–4901. [PubMed] [Google Scholar]
- 28.Specht JM, et al. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J Exp Med. 1997;186:1213–1221. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R-F, Appella E, Kawakami Y, Kang X, Rosenberg SA. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996;184:2207–2216. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Elsas A, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeh HJ, III, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 32.Overwijk WW, et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowne WB, et al. Coupling and uncoupling of tumor immunity and autoimmunity. J Exp Med. 1999;190:1717–1722. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludewig B, Odermatt B, Ochsenbein AF, Zinkernagel RM, Hengartner H. Role of dendritic cells in the induction and maintenance of autoimmune diseases. Immunol Rev. 1999;169:45–54. doi: 10.1111/j.1600-065x.1999.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 35.Minev B, McFarland BJ, Spiess PJ, Rosenberg SA, Restifo NP. Insertion signal sequence fused to minimal peptides elicits specific CD8+ T-cell responses and prolong survival of thymoma-bearing mice. Cancer Res. 1994;54:4155–4161. [PMC free article] [PubMed] [Google Scholar]
- 36.Minev BR, Chavez FL, Dudouet BM, Mitchell MS. Synthetic insertion signal sequences enhance MHC class I presentation of a peptide from the melanoma antigen MART-1. Eur J Immunol. 2000;30:2115–2124. doi: 10.1002/1521-4141(2000)30:8<2115::AID-IMMU2115>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 37.Wang R-F, Wang HY. Enhancement of antitumor immunity by prolonging antigen presentation on dendritic cells. Nat Biotechnol. 2002;20:149–156. doi: 10.1038/nbt0202-149. [DOI] [PubMed] [Google Scholar]
- 38.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frasca L, Piazza C, Piccolella E. CD4+ T cells orchestrate both amplification and deletion of CD8+ T cells. Crit Rev Immunol. 1998;18:569–594. doi: 10.1615/critrevimmunol.v18.i6.50. [DOI] [PubMed] [Google Scholar]
- 40.Pardoll DM, Topalian SL. The role of CD4(+) T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 41.Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 43.Schwarze SR, Dowdy SF. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol Sci. 2000;21:45–48. doi: 10.1016/s0165-6147(99)01429-7. [DOI] [PubMed] [Google Scholar]
- 44.Hung CF, et al. Improving vaccine potency through intercellular spreading and enhanced MHC class I presentation of antigen. J Immunol. 2001;166:5733–5740. doi: 10.4049/jimmunol.166.9.5733. [DOI] [PubMed] [Google Scholar]
- 45.Kim DT, et al. Introduction of soluble proteins into the MHC class I pathway by conjugation to an HIV tat peptide. J Immunol. 1997;159:1666–1668. [PubMed] [Google Scholar]
- 46.Srivastava PK. Immunotherapy of human cancer: lessons from mice. Nat Immunol. 2000;1:363–366. doi: 10.1038/808795. [DOI] [PubMed] [Google Scholar]
- 47.Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 48.Wang R-F. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001;22:269–276. doi: 10.1016/s1471-4906(01)01896-8. [DOI] [PubMed] [Google Scholar]
- 49.Schnell S, Young JW, Houghton AN, Sadelain M. Retrovirally transduced mouse dendritic cells require CD4+ T cell help to elicit antitumor immunity: implications for the clinical use of dendritic cells. J Immunol. 2000;164:1243–1250. doi: 10.4049/jimmunol.164.3.1243. [DOI] [PubMed] [Google Scholar]
- 50.Casares N, et al. Immunization with a tumor-associated CTL epitope plus a tumor-related or unrelated Th1 helper peptide elicits protective CTL immunity. Eur J Immunol. 2001;31:1780–1789. doi: 10.1002/1521-4141(200106)31:6<1780::aid-immu1780>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]