Abstract
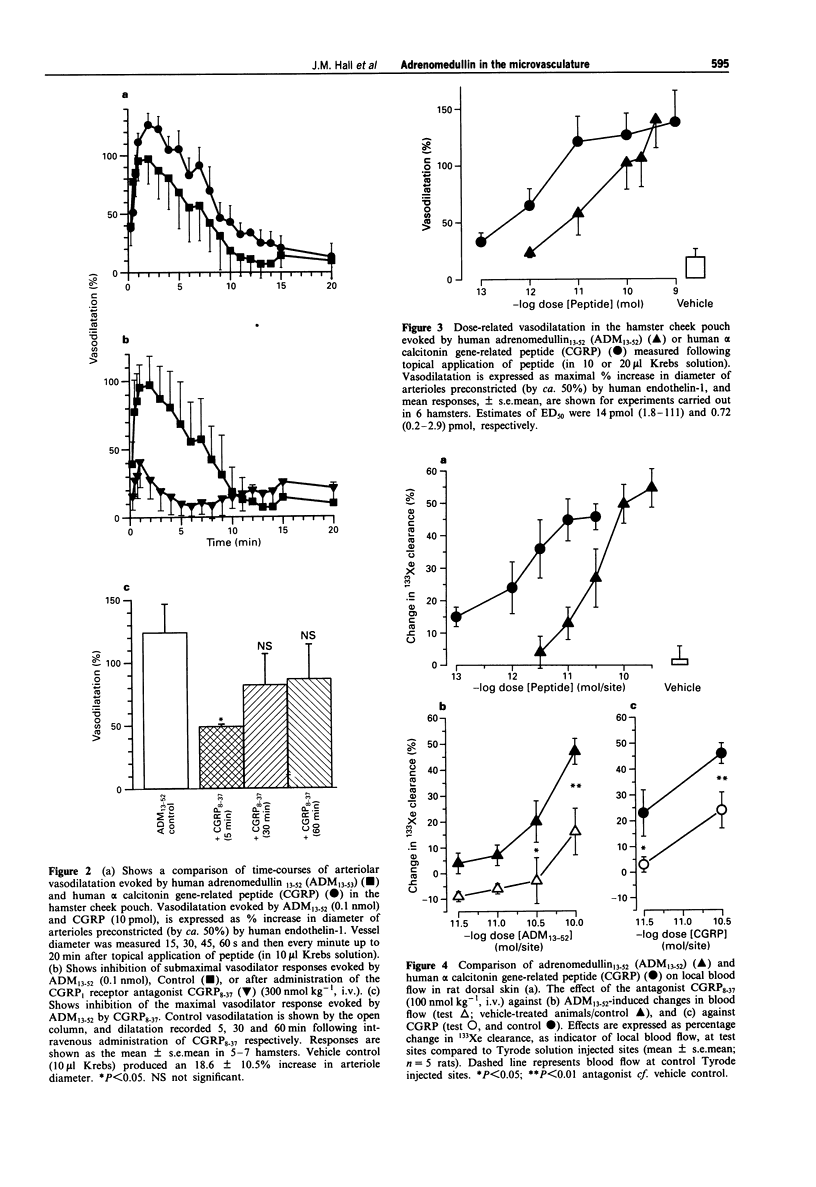
1. Adrenomedullin (ADM), a recently discovered circulating hypotensive peptide, shares limited sequence homology with the sensory nerve-derived vasodilator, calcitonin gene-related peptide (CGRP). This study compared the vasodilator effect of sequence 13-52 of human adrenomedullin (ADM13-52) with that of human alpha CGRP (CGRP), in the microvasculature of the hamster cheek pouch and rat skin in vivo. 2. Single arterioles (20-40 microns diameter) in the hamster cheek pouch were visualised by intravital microscopy and video recording, and measured by image analysis. Both ADM13-52 (1 pmol-0.4 nmol) and CGRP (0.1 pmol-1 nmol) evoked dose-related increases in the diameter of preconstricted arterioles (n = 6). ADM13-52 (ED50 14 pmol) was 20 fold less active than CGRP (ED50 0.71 pmol). The kinetics of onset and decline of vasodilator responses to both peptides were similar, with vasodilator responses to both peptides reaching a maximum at ca. 2 min, and reversing after 10-15 min (n = 5-7). The submaximal increase in blood flow evoked by ADM13-52 was significantly inhibited (P < 0.05; n = 6) by the CGRP1 receptor antagonist, CGRP8-37, at a dose (300 nmol kg-1, i.v.) that we have previously shown to inhibit significantly equivalent vasodilator responses to CGRP in this preparation. 3. In experiments measuring changes in local blood flow in rat skin by a 133xenon clearance technique, intradermal injection of both ADM13-52 (3-300 pmol) and CGRP (0.1-30 pmol) evoked dose-related increases in local blood flow.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brain S. D., Williams T. J., Tippins J. R., Morris H. R., MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985 Jan 3;313(5997):54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Chin S. Y., Hall J. M., Brain S. D., Morton I. K. Vasodilator responses to calcitonin gene-related peptide (CGRP) and amylin in the rat isolated perfused kidney are mediated via CGRP1 receptors. J Pharmacol Exp Ther. 1994 Jun;269(3):989–992. [PubMed] [Google Scholar]
- Claing A., Télémaque S., Cadieux A., Fournier A., Regoli D., D'Orléans-Juste P. Nonadrenergic and noncholinergic arterial dilatation and venoconstriction are mediated by calcitonin gene-related peptide1 and neurokinin-1 receptors, respectively, in the mesenteric vasculature of the rat after perivascular nerve stimulation. J Pharmacol Exp Ther. 1992 Dec;263(3):1226–1232. [PubMed] [Google Scholar]
- DeWitt B. J., Cheng D. Y., Caminiti G. N., Nossaman B. D., Coy D. H., Murphy W. A., Kadowitz P. J. Comparison of responses to adrenomedullin and calcitonin gene-related peptide in the pulmonary vascular bed of the cat. Eur J Pharmacol. 1994 May 23;257(3):303–306. doi: 10.1016/0014-2999(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Dennis T., Fournier A., Cadieux A., Pomerleau F., Jolicoeur F. B., St Pierre S., Quirion R. hCGRP8-37, a calcitonin gene-related peptide antagonist revealing calcitonin gene-related peptide receptor heterogeneity in brain and periphery. J Pharmacol Exp Ther. 1990 Jul;254(1):123–128. [PubMed] [Google Scholar]
- Duling B. R. The preparation and use of the hamster cheek pouch for studies of the microcirculation. Microvasc Res. 1973 May;5(3):423–429. doi: 10.1016/0026-2862(73)90059-9. [DOI] [PubMed] [Google Scholar]
- Eguchi S., Hirata Y., Kano H., Sato K., Watanabe Y., Watanabe T. X., Nakajima K., Sakakibara S., Marumo F. Specific receptors for adrenomedullin in cultured rat vascular smooth muscle cells. FEBS Lett. 1994 Mar 7;340(3):226–230. doi: 10.1016/0014-5793(94)80143-6. [DOI] [PubMed] [Google Scholar]
- Escott K. J., Brain S. D. Effect of a calcitonin gene-related peptide antagonist (CGRP8-37) on skin vasodilatation and oedema induced by stimulation of the rat saphenous nerve. Br J Pharmacol. 1993 Oct;110(2):772–776. doi: 10.1111/j.1476-5381.1993.tb13878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani S., Wimalawansa S. J., Maggi C. A. Involvement of multiple receptors in the biological effects of calcitonin gene-related peptide and amylin in rat and guinea-pig preparations. Br J Pharmacol. 1992 Oct;107(2):510–514. doi: 10.1111/j.1476-5381.1992.tb12775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. M., Brain S. D. Inhibition by SR 140333 of NK1 tachykinin receptor-evoked, nitric oxide-dependent vasodilatation in the hamster cheek pouch microvasculature in vivo. Br J Pharmacol. 1994 Oct;113(2):522–526. doi: 10.1111/j.1476-5381.1994.tb17020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q., Chang J. K., Gharavi H., Fortenberry Y., Hyman A., Lippton H. An adrenomedullin (ADM) fragment retains the systemic vasodilator activity of human ADM. Life Sci. 1994;54(16):PL265–PL270. doi: 10.1016/0024-3205(94)00844-2. [DOI] [PubMed] [Google Scholar]
- Ichiki Y., Kitamura K., Kangawa K., Kawamoto M., Matsuo H., Eto T. Distribution and characterization of immunoreactive adrenomedullin in human tissue and plasma. FEBS Lett. 1994 Jan 24;338(1):6–10. doi: 10.1016/0014-5793(94)80106-1. [DOI] [PubMed] [Google Scholar]
- Ishiyama Y., Kitamura K., Ichiki Y., Nakamura S., Kida O., Kangawa K., Eto T. Hemodynamic effects of a novel hypotensive peptide, human adrenomedullin, in rats. Eur J Pharmacol. 1993 Sep 14;241(2-3):271–273. doi: 10.1016/0014-2999(93)90214-3. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Ichiki Y., Tanaka M., Kawamoto M., Emura J., Sakakibara S., Kangawa K., Matsuo H., Eto T. Immunoreactive adrenomedullin in human plasma. FEBS Lett. 1994 Mar 21;341(2-3):288–290. doi: 10.1016/0014-5793(94)80474-5. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Kangawa K., Kawamoto M., Ichiki Y., Nakamura S., Matsuo H., Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993 Apr 30;192(2):553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Kangawa K., Kojima M., Ichiki Y., Matsuo H., Eto T. Complete amino acid sequence of porcine adrenomedullin and cloning of cDNA encoding its precursor. FEBS Lett. 1994 Feb 7;338(3):306–310. doi: 10.1016/0014-5793(94)80289-0. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Sakata J., Kangawa K., Kojima M., Matsuo H., Eto T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun. 1993 Jul 30;194(2):720–725. doi: 10.1006/bbrc.1993.1881. [DOI] [PubMed] [Google Scholar]
- Lawrence E., Brain S. D. Responses to endothelins in the rat cutaneous microvasculature: a modulatory role of locally-produced nitric oxide. Br J Pharmacol. 1992 Jul;106(3):733–738. doi: 10.1111/j.1476-5381.1992.tb14402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippton H., Chang J. K., Hao Q., Summer W., Hyman A. L. Adrenomedullin dilates the pulmonary vascular bed in vivo. J Appl Physiol (1985) 1994 May;76(5):2154–2156. doi: 10.1152/jappl.1994.76.5.2154. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Rovero P., Giuliani S., Evangelista S., Regoli D., Meli A. Biological activity of N-terminal fragments of calcitonin gene-related peptide. Eur J Pharmacol. 1990 Apr 10;179(1-2):217–219. doi: 10.1016/0014-2999(90)90422-3. [DOI] [PubMed] [Google Scholar]
- Nuki C., Kawasaki H., Kitamura K., Takenaga M., Kangawa K., Eto T., Wada A. Vasodilator effect of adrenomedullin and calcitonin gene-related peptide receptors in rat mesenteric vascular beds. Biochem Biophys Res Commun. 1993 Oct 15;196(1):245–251. doi: 10.1006/bbrc.1993.2241. [DOI] [PubMed] [Google Scholar]
- Perret M., Broussard H., LeGros T., Burns A., Chang J. K., Summer W., Hyman A., Lippton H. The effect of adrenomedullin on the isolated heart. Life Sci. 1993;53(22):PL377–PL379. doi: 10.1016/0024-3205(93)90213-m. [DOI] [PubMed] [Google Scholar]
- Sakata J., Shimokubo T., Kitamura K., Nakamura S., Kangawa K., Matsuo H., Eto T. Molecular cloning and biological activities of rat adrenomedullin, a hypotensive peptide. Biochem Biophys Res Commun. 1993 Sep 15;195(2):921–927. doi: 10.1006/bbrc.1993.2132. [DOI] [PubMed] [Google Scholar]
- Tippins J. R., Di Marzo V., Panico M., Morris H. R., MacIntyre I. Investigation of the structure/activity relationship of human calcitonin gene-related peptide (CGRP). Biochem Biophys Res Commun. 1986 Feb 13;134(3):1306–1311. doi: 10.1016/0006-291x(86)90392-x. [DOI] [PubMed] [Google Scholar]
- Williams T. J. Proceedings:Simultaneous measurement of local plasma exudation and blood flow changes induced by intradermal injection of vasoactive substances, using [131I]albumen and 133Xe. J Physiol. 1976 Jan;254(1):4P–5P. [PubMed] [Google Scholar]
- Zaidi M., Brain S. D., Tippins J. R., Di Marzo V., Moonga B. S., Chambers T. J., Morris H. R., MacIntyre I. Structure-activity relationship of human calcitonin-gene-related peptide. Biochem J. 1990 Aug 1;269(3):775–780. doi: 10.1042/bj2690775. [DOI] [PMC free article] [PubMed] [Google Scholar]


