Abstract
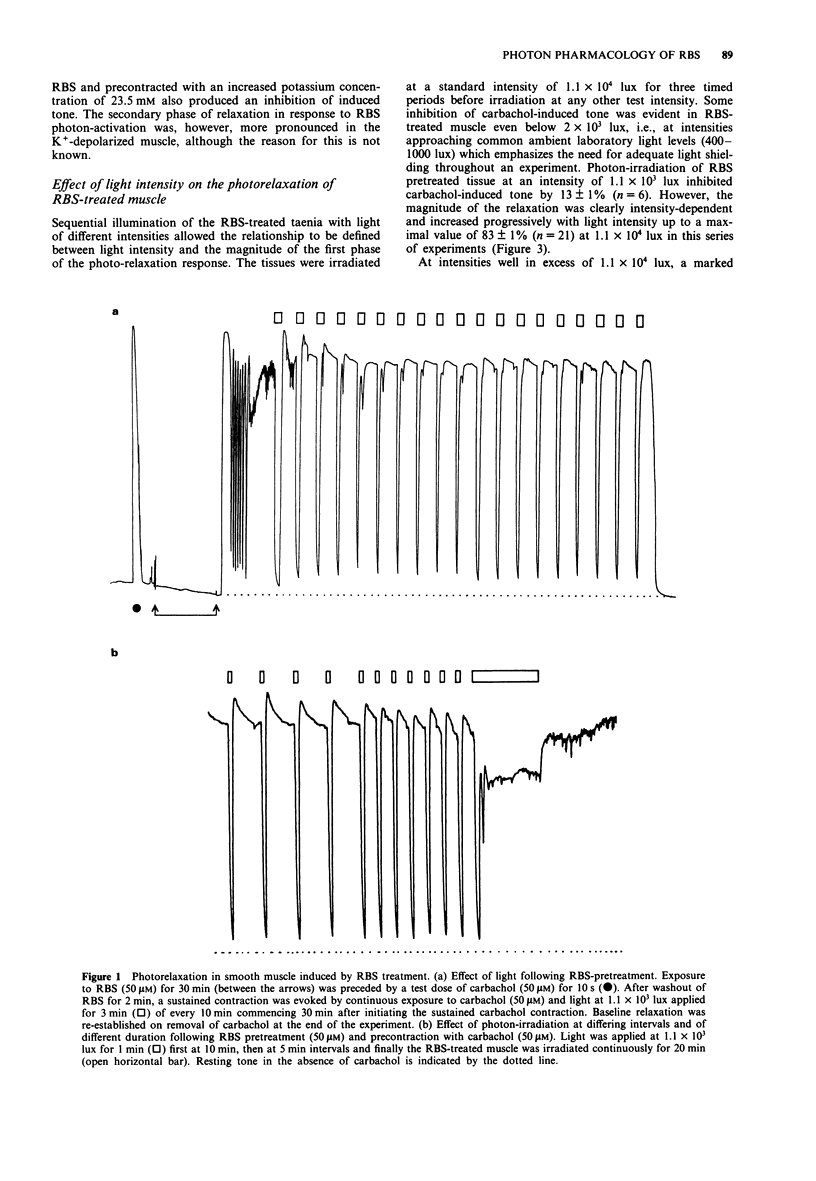
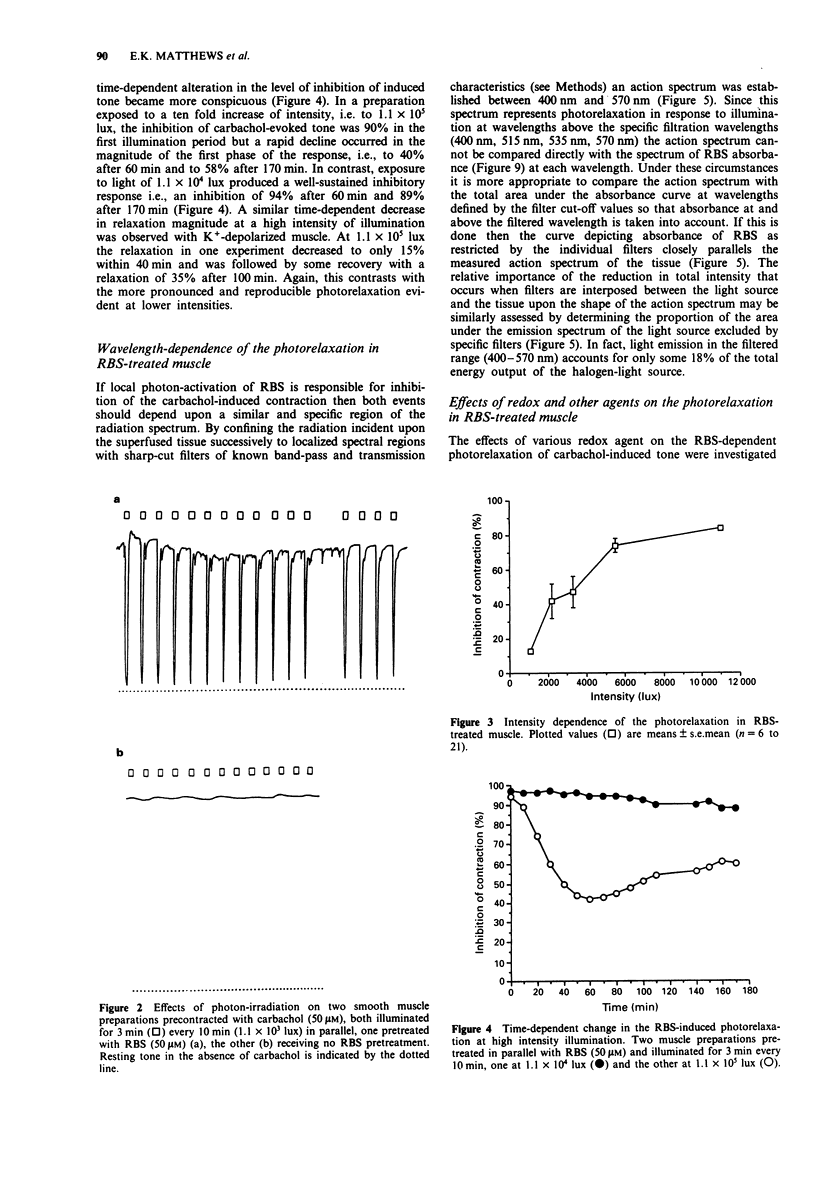
1. The mechanisms of action on smooth muscle of the iron-sulphur cluster nitrosyl compound, heptanitrosyl-tri-mu 3-thioxotetraferrate (1-), (RBS), a photosensitive nitric oxide donor, have been investigated in the guinea-pig taenia caeci (coli) in vitro. 2. After exposure to RBS (50 microM) for 30 min, and subsequent washout, a sustained contraction was recorded in the absence of light to either the agonist carbachol (50 microM) or a depolarizing concentration of KCl (23.5 mM). Photon irradiation (> 400 nm) caused a prompt relaxation of precontracted RBS-treated muscle, the magnitude of which depended upon the intensity (1.1 x 10(3) to 1.1 x 10(5) lux), duration (30 s to 20 min) and wavelength (400 to 800 nm), of the incident illumination. 3. Repeated periods of illumination at 1.1 x 10(4) lux produced a reversible relaxation of both carbachol and KCl-evoked tone in muscle pretreated with RBS (50 microM). These photorelaxations were reproducible at 10 min intervals for several hours with a maximal relaxation amounting to 80 to 90% that of the tone produced by carbachol (50 microM). 4. The nitric oxide synthase inhibitor, NG-nitro-L-arginine (60 microM), caused no inhibition of the photon-induced relaxation of RBS-treated muscle. In contrast, N-methylhydroxylamine (2 mM), L-cysteine (10 mM), DL-dithiothreitol (2 mM), methylene blue (30 microM), and haemoglobin (20 microM), all reversibly but significantly inhibited (P < 0.001) the photorelaxation response. However, neither the aminothiol N-acetyl-L-cysteine (10 mM) nor the tripeptide glutathione (10 mM) blocked the RBS-induced photorelaxation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

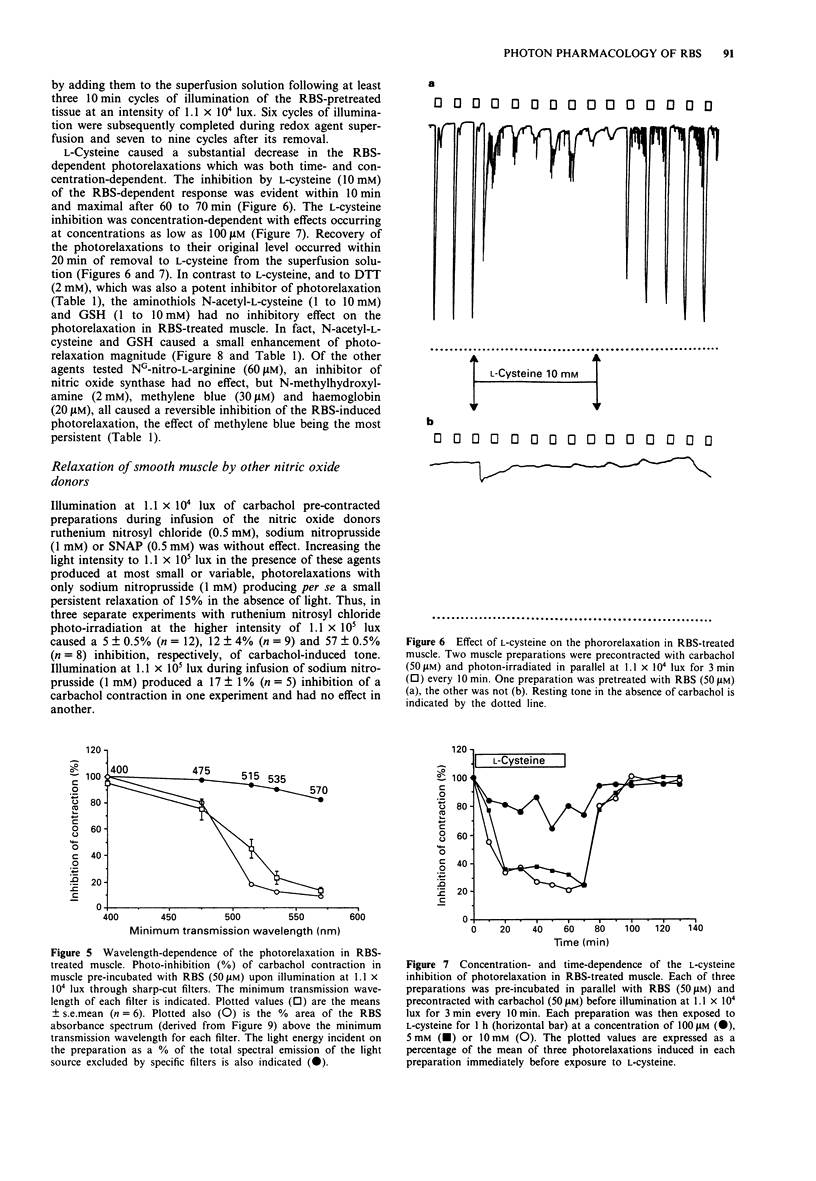
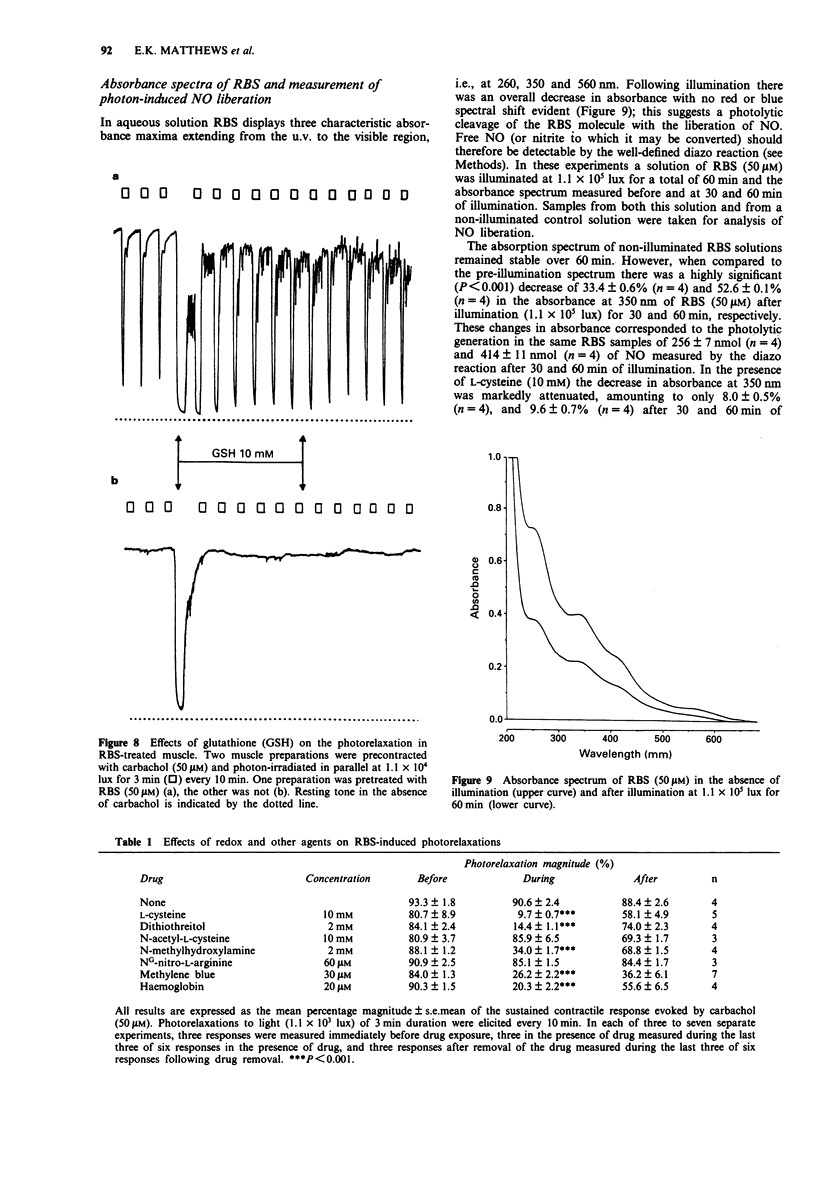


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannai S. Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta. 1984 Sep 3;779(3):289–306. doi: 10.1016/0304-4157(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braughler J. M. Soluble guanylate cyclase activation by nitric oxide and its reversal. Involvement of sulfhydryl group oxidation and reduction. Biochem Pharmacol. 1983 Mar 1;32(5):811–818. doi: 10.1016/0006-2952(83)90581-6. [DOI] [PubMed] [Google Scholar]
- Flitney F. W., Megson I. L., Flitney D. E., Butler A. R. Iron-sulphur cluster nitrosyls, a novel class of nitric oxide generator: mechanism of vasodilator action on rat isolated tail artery. Br J Pharmacol. 1992 Nov;107(3):842–848. doi: 10.1111/j.1476-5381.1992.tb14534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Vanhoutte P. M. Attenuation of contractions to acetylcholine in canine bronchi by an endogenous nitric oxide-like substance. Br J Pharmacol. 1993 Jul;109(3):887–891. doi: 10.1111/j.1476-5381.1993.tb13658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Babbedge R., Brave S. R., Hart S. L., Hobbs A. J., Tucker J. F., Wallace P., Moore P. K. An investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br J Pharmacol. 1992 Nov;107(3):715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S. N-methylhydroxylamine inhibits and M&B 22948 potentiates relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs. Br J Pharmacol. 1989 Mar;96(3):637–644. doi: 10.1111/j.1476-5381.1989.tb11863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. J., Gibson A. L-NG-nitro-arginine and its methyl ester are potent inhibitors of non-adrenergic, non-cholinergic transmission in the rat anococcygeus. Br J Pharmacol. 1990 Aug;100(4):749–752. doi: 10.1111/j.1476-5381.1990.tb14086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S., Arnold W. P., Murad F. Effects of sodium nitroprusside, nitroglycerin, and sodium azide on levels of cyclic nucleotides and mechanical activity of various tissues. J Cyclic Nucleotide Res. 1977 Aug;3(4):239–247. [PubMed] [Google Scholar]
- Matsunaga K., Furchgott R. F. Interactions of light and sodium nitrite in producing relaxation of rabbit aorta. J Pharmacol Exp Ther. 1989 Feb;248(2):687–695. [PubMed] [Google Scholar]
- Matthews E. K., Flaherty C., Smith W. H. Photodynamic action of aluminium phthalocyanine tetrasulphonate (A1PcS4) on smooth muscle: effects of thiols and a cyclic GMP analogue. Br J Pharmacol. 1993 Nov;110(3):1248–1254. doi: 10.1111/j.1476-5381.1993.tb13949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Mesler D. E. Photodynamic effects of erythrosine on the smooth muscle cells of guinea-pig taenia coli. Br J Pharmacol. 1984 Oct;83(2):555–566. doi: 10.1111/j.1476-5381.1984.tb16520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B., Brunner F., Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol. 1993 Jan 26;45(2):367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Grossman M. J. Novel iron-sulfur clusters. Trends Biochem Sci. 1993 May;18(5):153–154. doi: 10.1016/0968-0004(93)90101-r. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]


