Abstract
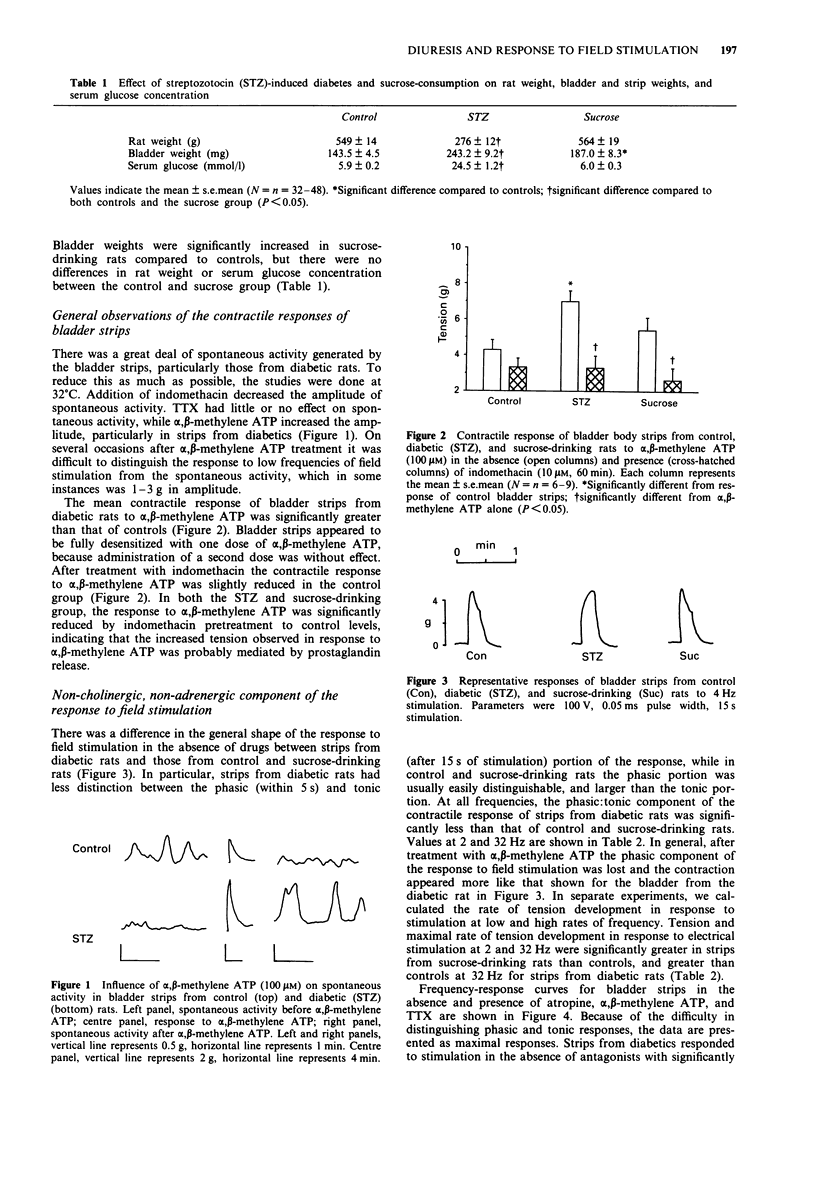
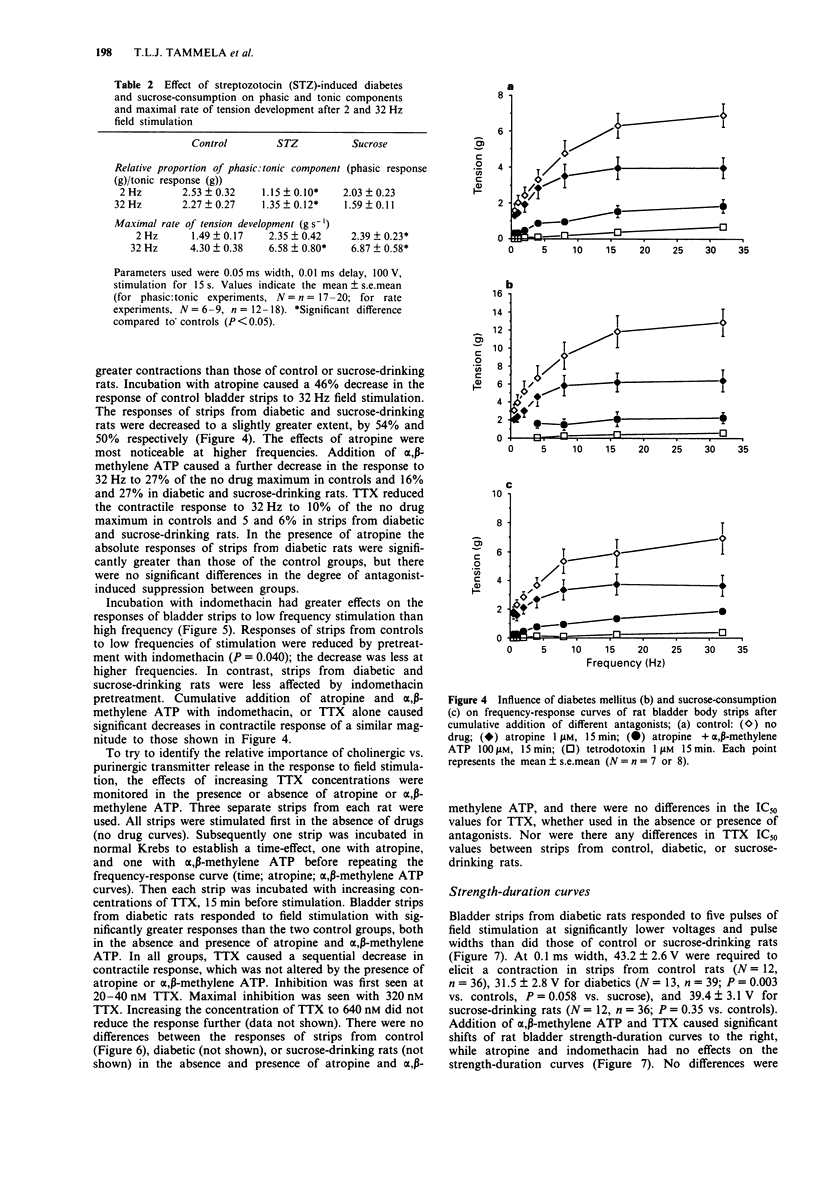
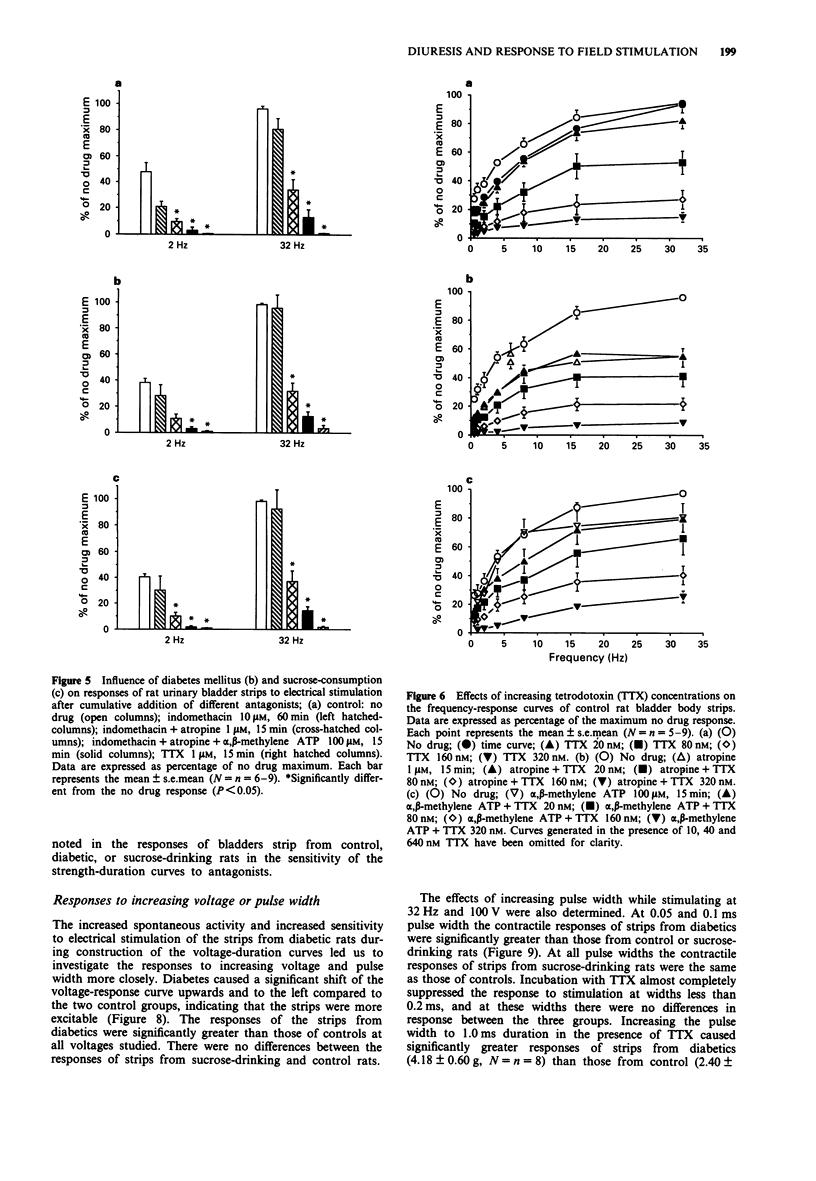
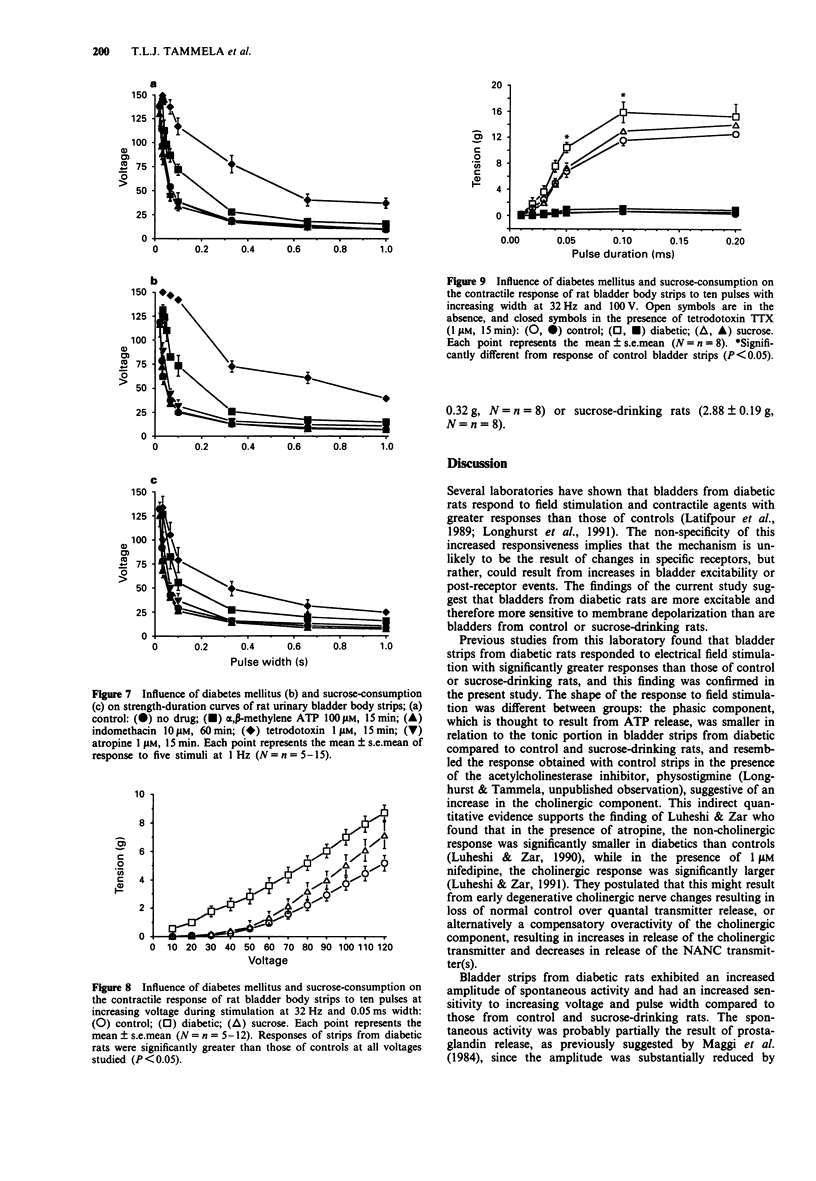
1. The responses of bladder strips from control, streptozotocin-diabetic, and sucrose-drinking rats to electrical field stimulation were investigated. Sucrose-drinking rats were included as additional controls because they have enlarged bladders as a result of non-diabetic diuresis. 2. Bladder strips from diabetic rats developed more spontaneous activity than those from the two control groups. Indomethacin reduced the amplitude and frequency of spontaneous contractions suggesting that they resulted from endogenous prostaglandin formation. Tetrodotoxin (TTX) had little effect, while alpha, beta-methylene ATP caused increases in spontaneous activity. 3. Bladder strips from diabetic rats responded to field stimulation with greater contractions than controls in the absence of antagonists as well as in the presence of atropine and alpha, beta-methylene ATP. Increasing TTX concentrations caused a step-wise depression of the contractile response to electrical stimulation which was not affected by preincubation with either atropine or alpha, beta-methylene ATP. 4. Atropine and indomethacin had no effect on strength-duration curves constructed to measure threshold contractile responses to five pulses stimulation. The curves were shifted to the right by both TTX and alpha, beta-methylene ATP, indicating that the responses were neurogenic in nature and at least partially, the result of stimulation of P2-purinoceptors. In the absence of drugs, bladder strips from diabetics responded at lower voltages and pulse widths than those of control and sucrose-drinking rats, suggesting that they were more excitable. 5. The response curve of bladder strips from diabetics to field stimulation at increasing voltage was shifted upwards and to the left compared to strips from control or sucrose-drinking rats.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. F. Evidence for a prostaglandin link in the purinergic activation of rabbit bladder smooth muscle. J Pharmacol Exp Ther. 1982 Feb;220(2):347–352. [PubMed] [Google Scholar]
- Anderson G. F., Kohn K. I. Interactions of calcium, prostaglandins and indomethacin on the smooth muscle of the bladder. Pharmacology. 1978;16(6):306–313. doi: 10.1159/000136786. [DOI] [PubMed] [Google Scholar]
- Andersson K. E., Husted S., Sjögren C. Contribution of prostaglandins to the adenosine triphosphate-induced contraction of rabbit urinary bladder. Br J Pharmacol. 1980 Nov;70(3):443–452. doi: 10.1111/j.1476-5381.1980.tb08722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson P. O., Malmgren A., Uvelius B. Cystometrical and in vitro evaluation of urinary bladder function in rats with streptozotocin-induced diabetes. J Urol. 1988 Jun;139(6):1359–1362. doi: 10.1016/s0022-5347(17)42919-3. [DOI] [PubMed] [Google Scholar]
- Barkai L., Szabó L. Urinary bladder dysfunction in diabetic children with and without subclinical cardiovascular autonomic neuropathy. Eur J Pediatr. 1993 Mar;152(3):190–192. doi: 10.1007/BF01956141. [DOI] [PubMed] [Google Scholar]
- Belis J. A., Curley R. M., Murty V. N., Wagner C. H., Winter S. J., Rohner T. J., Jr Calcium channel agonist/antagonist effects on cholinergic stimulation of the diabetic rat bladder. Pharmacology. 1992;44(2):81–91. doi: 10.1159/000138876. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Williams J. H. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and alpha,beta-methylene ATP. Br J Pharmacol. 1990 Mar;99(3):493–498. doi: 10.1111/j.1476-5381.1990.tb12956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter F. G. Impairment and restoration of rat urinary bladder responsiveness following distension. Am J Physiol. 1983 Jan;244(1):R106–R113. doi: 10.1152/ajpregu.1983.244.1.R106. [DOI] [PubMed] [Google Scholar]
- Choo L. K., Mitchelson F. The effect of indomethacin and adenosine 5'-triphosphate on the excitatory innervation of the rate urinary bladder. Can J Physiol Pharmacol. 1980 Sep;58(9):1042–1048. doi: 10.1139/y80-157. [DOI] [PubMed] [Google Scholar]
- Dean D. M., Downie J. W. Interaction of prostaglandins and adenosine 5'-triphosphate in the noncholinergic neurotransmission in rabbit detrusor. Prostaglandins. 1978 Aug;16(2):245–251. doi: 10.1016/0090-6980(78)90026-6. [DOI] [PubMed] [Google Scholar]
- Eika B., Levin R. M., Longhurst P. A. Collagen and bladder function in streptozotocin-diabetic rats: effects of insulin and aminoguanidine. J Urol. 1992 Jul;148(1):167–172. doi: 10.1016/s0022-5347(17)36546-1. [DOI] [PubMed] [Google Scholar]
- Ekström J. Increase in choline acetyltransferase activity in surgically isolated postganglionic parasympathetic neurones of the urinary bladder of adult rats. Acta Physiol Scand. 1981 Jan;111(1):81–86. doi: 10.1111/j.1748-1716.1981.tb06708.x. [DOI] [PubMed] [Google Scholar]
- Ekström J., Malmberg L. Development of supersensitivity to methacholine in the rat detrusor following either parasympathetic denervation or decentralization. Acta Physiol Scand. 1984 Oct;122(2):175–179. doi: 10.1111/j.1748-1716.1984.tb07495.x. [DOI] [PubMed] [Google Scholar]
- Ekström J., Uvelius B. Length-tension relations of smooth muscle from normal and denervated rat urinary bladders. Acta Physiol Scand. 1981 Aug;112(4):443–447. doi: 10.1111/j.1748-1716.1981.tb06842.x. [DOI] [PubMed] [Google Scholar]
- Faerman I., Glocer L., Celener D., Jadzinsky M., Fox D., Maler M., Alvarez E. Autonomic nervous system and diabetes. Histological and histochemical study of the autonomic nerve fibers of the urinary bladder in diabetic patients. Diabetes. 1973 Apr;22(4):225–237. doi: 10.2337/diab.22.4.225. [DOI] [PubMed] [Google Scholar]
- Faerman I., Maler M., Jadzinsky M., Alvarez E., Fox D., Zilbervarg J., Cibeira J. B., Colinas R. Asymptomatic neurogenic bladder in juvenile diabetics. Diabetologia. 1971 Jun;7(3):168–172. doi: 10.1007/BF01212549. [DOI] [PubMed] [Google Scholar]
- Fleming W. W., McPhillips J. J., Westfall D. P. Postjunctional supersensitivity and subsensitivity of excitable tissues to drugs. Ergeb Physiol. 1973;68:55–119. doi: 10.1007/3-540-06238-6_5. [DOI] [PubMed] [Google Scholar]
- Fleming W. W., Westfall D. P. Altered resting membrane potential in the supersensitive vas deferens of the guinea pig. J Pharmacol Exp Ther. 1975 Feb;192(2):381–389. [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Are disturbances of sorbitol, phosphoinositide, and Na+-K+-ATPase regulation involved in pathogenesis of diabetic neuropathy? Diabetes. 1988 Jun;37(6):688–693. doi: 10.2337/diab.37.6.688. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Mikhailidis D. P., Thompson C. S., Dandona P. The effect of streptozotocin-induced diabetes on PGI2 synthesis by the rat bladder. J Urol. 1986 Jun;135(6):1290–1292. doi: 10.1016/s0022-5347(17)46076-9. [DOI] [PubMed] [Google Scholar]
- Kudlacz E. M., Gerald M. C., Wallace L. J. Effects of diabetes and diuresis on contraction and relaxation mechanisms in rat urinary bladder. Diabetes. 1989 Mar;38(3):278–284. doi: 10.2337/diab.38.3.278. [DOI] [PubMed] [Google Scholar]
- Latifpour J., Gousse A., Kondo S., Morita T., Weiss R. M. Effects of experimental diabetes on biochemical and functional characteristics of bladder muscarinic receptors. J Pharmacol Exp Ther. 1989 Jan;248(1):81–88. [PubMed] [Google Scholar]
- Lincoln J., Crockett M., Haven A. J., Burnstock G. Rat bladder in the early stages of streptozotocin-induced diabetes: adrenergic and cholinergic innervation. Diabetologia. 1984 Jan;26(1):81–87. doi: 10.1007/BF00252269. [DOI] [PubMed] [Google Scholar]
- Longhurst P. A., Brotcke T. P., Leggett R. E., Levin R. M. The influence of streptozotocin-induced diabetes mellitus on the sensitivity of rat urinary bladder body and base strips to changes in extracellular calcium. Gen Pharmacol. 1992 Jan;23(1):83–88. doi: 10.1016/0306-3623(92)90052-l. [DOI] [PubMed] [Google Scholar]
- Longhurst P. A., Kang J., Wein A. J., Levin R. M. Length-tension relationship of urinary bladder strips from streptozotocin-diabetic rats. Pharmacology. 1990;40(2):110–121. doi: 10.1159/000138649. [DOI] [PubMed] [Google Scholar]
- Longhurst P. A., Kauer J., Levin R. M. The ability of insulin treatment to reverse or prevent the changes in urinary bladder function caused by streptozotocin-induced diabetes mellitus. Gen Pharmacol. 1991;22(2):305–311. doi: 10.1016/0306-3623(91)90454-e. [DOI] [PubMed] [Google Scholar]
- Luheshi G. N., Zar M. A. Inhibitory effect of streptozotocin-induced diabetes on non-cholinergic motor transmission in rat detrusor and its prevention by sorbinil. Br J Pharmacol. 1990 Oct;101(2):411–417. doi: 10.1111/j.1476-5381.1990.tb12723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luheshi G. N., Zar M. A. The effect of streptozotocin-induced diabetes on cholinergic motor transmission in the rat urinary bladder. Br J Pharmacol. 1991 Jul;103(3):1657–1662. doi: 10.1111/j.1476-5381.1991.tb09843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Evangelista S., Grimaldi G., Santicioli P., Giolitti A., Meli A. Evidence for the involvement of arachidonic acid metabolites in spontaneous and drug-induced contractions of rat urinary bladder. J Pharmacol Exp Ther. 1984 Aug;230(2):500–513. [PubMed] [Google Scholar]
- Moller C. F. Diabetic cystopathy.I: A clinical study of the frequency of bladder dysfunction in diabetics. Dan Med Bull. 1976 Dec;23(6):267–278. [PubMed] [Google Scholar]
- Nadelhaft I., Vera P. L. Reduced urinary bladder afferent conduction velocities in streptozocin diabetic rats. Neurosci Lett. 1992 Feb 3;135(2):276–278. doi: 10.1016/0304-3940(92)90455-g. [DOI] [PubMed] [Google Scholar]
- Paro M., Italiano G., Travagli R. A., Petrelli L., Zanoni R., Prosdocimi M., Fiori M. G. Cystometric changes in alloxan diabetic rats: evidence for functional and structural correlates of diabetic autonomic neuropathy. J Auton Nerv Syst. 1990 Apr;30(1):1–11. doi: 10.1016/0165-1838(90)90158-f. [DOI] [PubMed] [Google Scholar]
- Pinna C., Caratozzolo O., Puglisi L. A possible role for urinary bladder epithelium in bradykinin-induced contraction in diabetic rats. Eur J Pharmacol. 1992 Apr 22;214(2-3):143–148. doi: 10.1016/0014-2999(92)90111-g. [DOI] [PubMed] [Google Scholar]
- Posangi J., Zar M. A., Harris J. B. The action of palytoxin on the isolated detrusor muscle of the rat. Br J Pharmacol. 1992 Jun;106(2):307–314. doi: 10.1111/j.1476-5381.1992.tb14333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santicioli P., Gamse R., Maggi C. A., Meli A. Cystometric changes in the early phase of streptozotocin-induced diabetes in rats: evidence for sensory changes not correlated to diabetic neuropathy. Naunyn Schmiedebergs Arch Pharmacol. 1987 May;335(5):580–587. doi: 10.1007/BF00169128. [DOI] [PubMed] [Google Scholar]
- Sibley G. N. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol. 1984 Sep;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steers W. D., Mackway A. M., Ciambotti J., de Groat W. C. Effects of streptozotocin-induced diabetes on bladder function in the rat. J Urol. 1990 May;143(5):1032–1036. doi: 10.1016/s0022-5347(17)40177-7. [DOI] [PubMed] [Google Scholar]


