Abstract
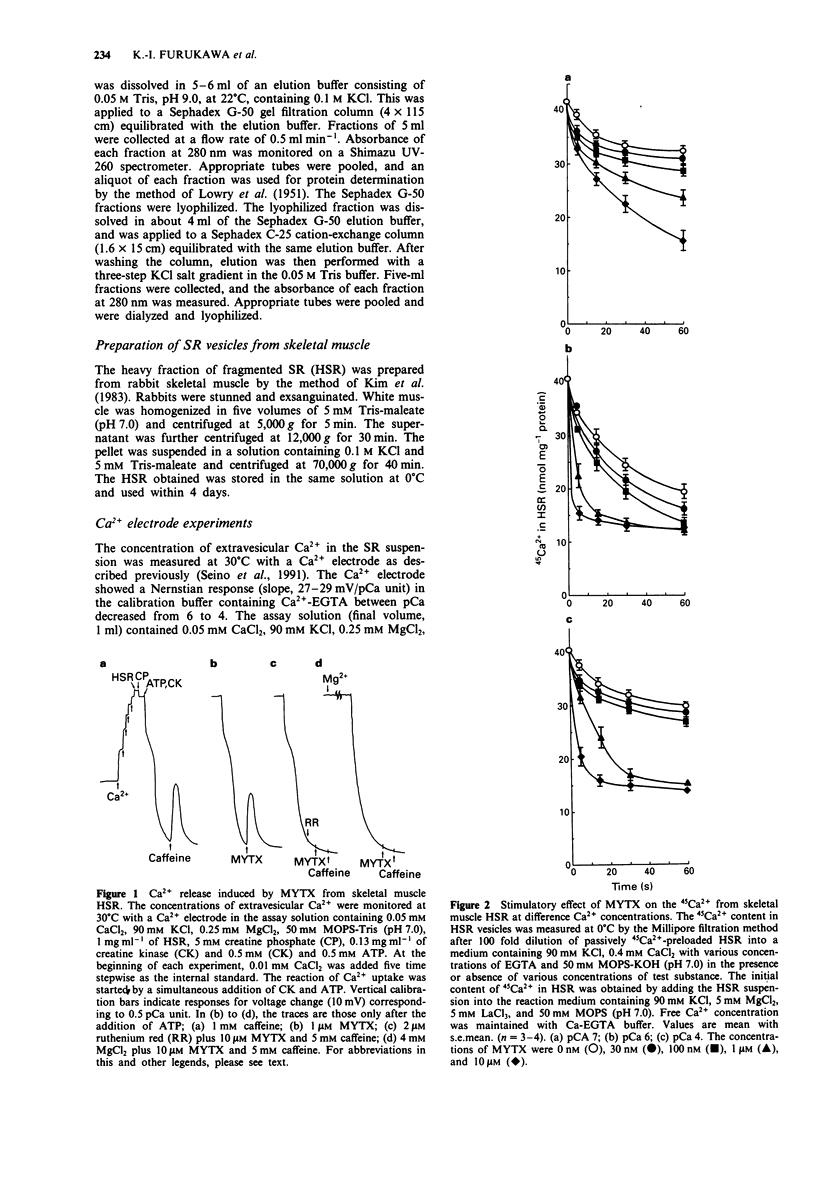
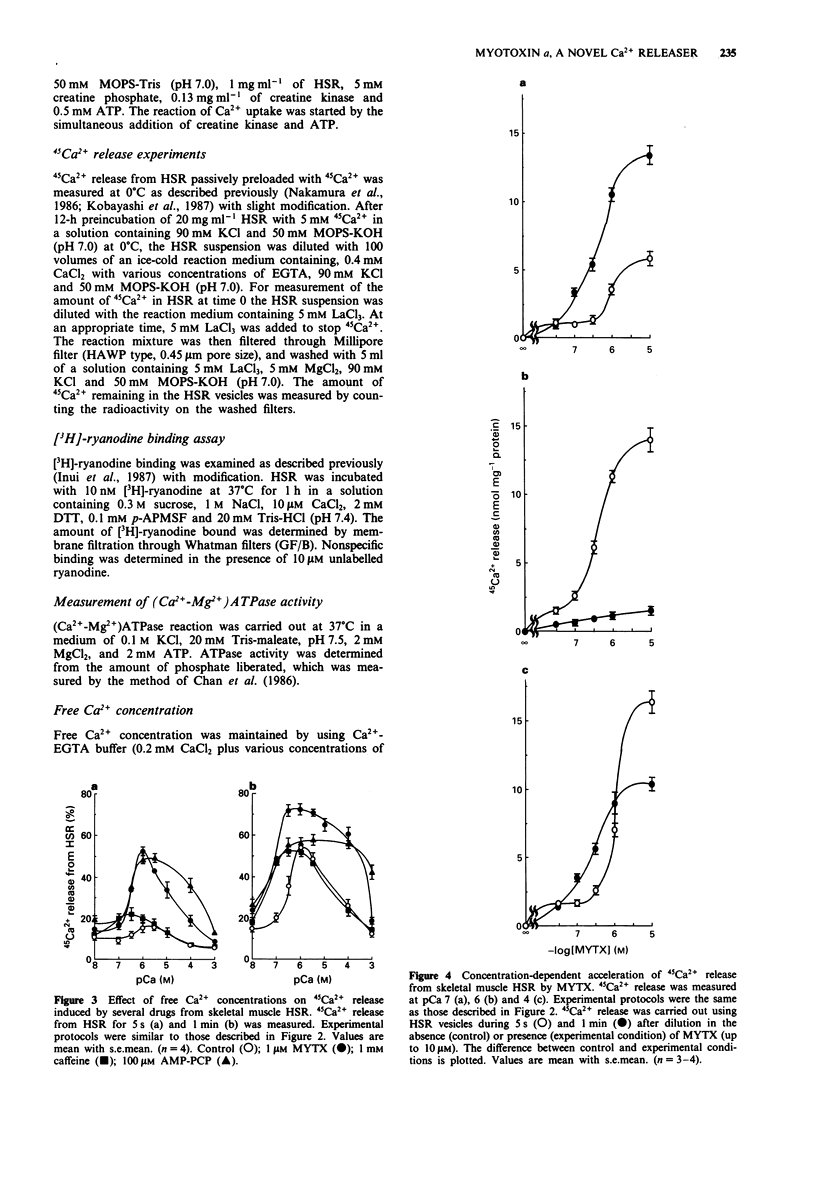
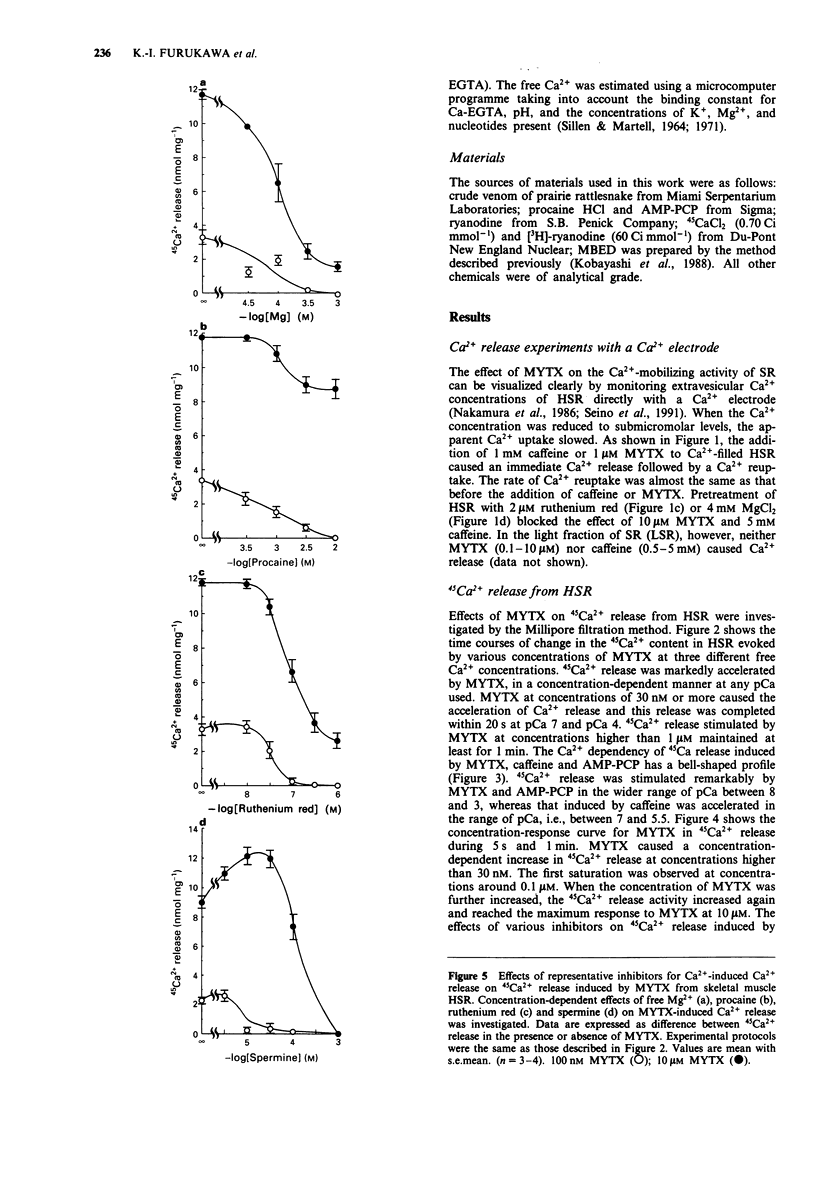
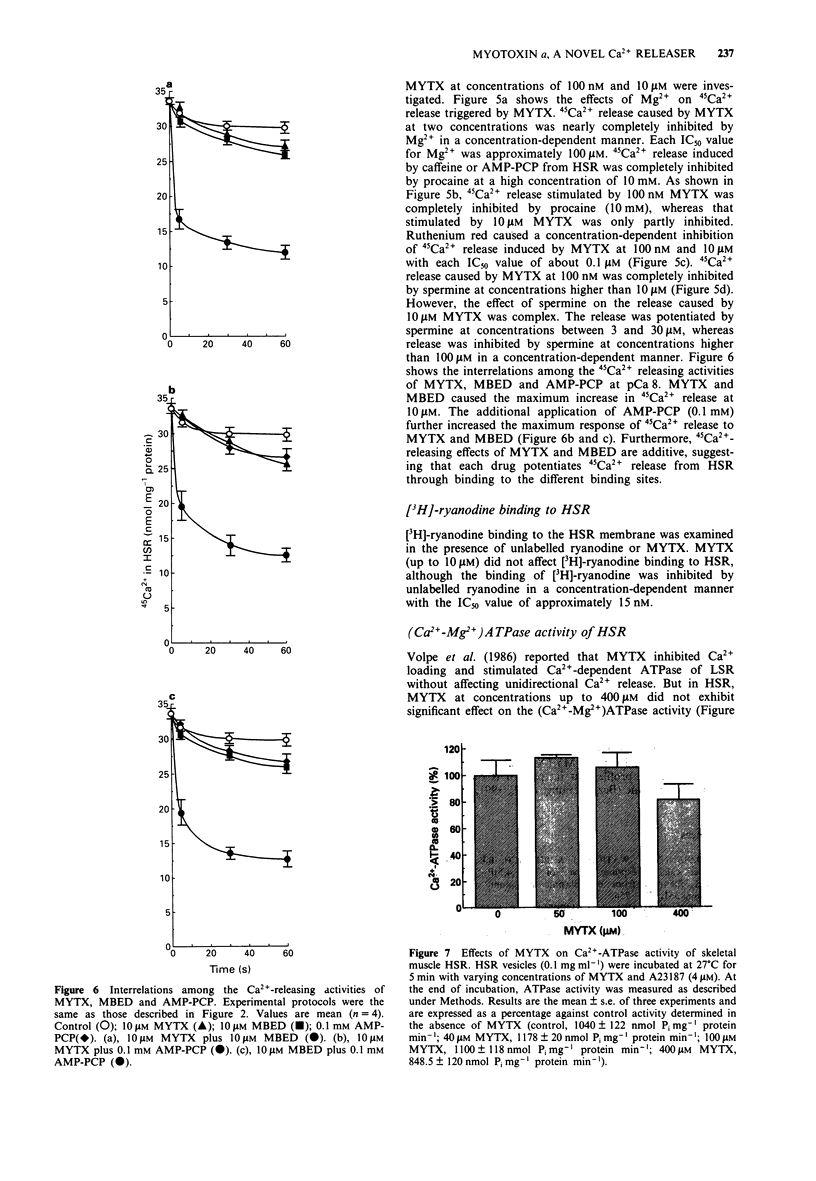
1. Myotoxin alpha (MYTX), a polypeptide toxin purified from the venom of prairie rattlesnakes (Crotalus viridis viridis) induced Ca2+ release from the heavy fraction (HSR) but not the light fraction of skeletal sarcoplasmic reticulum at concentrations higher than 1 microM, followed by spontaneous Ca2+ reuptake by measuring extravesicular Ca2+ concentrations using the Ca2+ electrode. 2. The rate of 45Ca2+ release from HSR vesicles was markedly accelerated by MYTX in a concentration-dependent manner in the range of concentrations between 30 nM and 10 microM, indicating the most potent Ca2+ releaser in HSR. 3. The Ca2+ dependency of MYTX-induced 45Ca2+ release has a bell-shaped profile but it was quite different from that of caffeine, an inducer of Ca(2+)-induced Ca2+ release. 4. 45Ca2+ release induced by MYTX was remarkable in the range of pCa between 8 and 3, whereas that by caffeine was prominent in the range of pCa, i.e., between 7 and 5.5. 5. MYTX-induced 45Ca2+ release consists of both early and late components. The early component caused by MYTX at low concentrations (30-300 nM) completed within 20 s, while the late component induced by it at higher concentrations (> 0.3 microM) was maintained for at least 1 min. 6. Both the components were almost completely inhibited by inhibitors of Ca2+ such as Mg2+, ruthenium red and spermine. 7. 45Ca2+ release induced by caffeine or beta,gamma-methyleneadenosine 5'-triphosphate (AMP-PCP) was completely inhibited by high concentrations of procaine. Procaine abolished the early component but not the late one, suggesting that at least the early component is mediated through Ca(2+)-induced Ca2+ release channels.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. M., Delfert D., Junger K. D. A direct colorimetric assay for Ca2+ -stimulated ATPase activity. Anal Biochem. 1986 Sep;157(2):375–380. doi: 10.1016/0003-2697(86)90640-8. [DOI] [PubMed] [Google Scholar]
- Ebashi S. Excitation-contraction coupling and the mechanism of muscle contraction. Annu Rev Physiol. 1991;53:1–16. doi: 10.1146/annurev.ph.53.030191.000245. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fang Y. I., Adachi M., Kobayashi J., Ohizumi Y. High affinity binding of 9-[3H]methyl-7-bromoeudistomin D to the caffeine-binding site of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1993 Sep 5;268(25):18622–18625. [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Fox J. W., Elzinga M., Tu A. T. Amino acid sequence and disulfide bond assignment of myotoxin a isolated from the venom of Prairie rattlesnake (Crotalus viridis viridis). Biochemistry. 1979 Feb 20;18(4):678–684. doi: 10.1021/bi00571a020. [DOI] [PubMed] [Google Scholar]
- Hymel L., Inui M., Fleischer S., Schindler H. Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers. Proc Natl Acad Sci U S A. 1988 Jan;85(2):441–445. doi: 10.1073/pnas.85.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Kim D. H., Ohnishi S. T., Ikemoto N. Kinetic studies of calcium release from sarcoplasmic reticulum in vitro. J Biol Chem. 1983 Aug 25;258(16):9662–9668. [PubMed] [Google Scholar]
- Kobayashi J., Taniguchi M., Hino T., Ohizumi Y. Eudistomin derivatives, novel phosphodiesterase inhibitors: synthesis and relative activity. J Pharm Pharmacol. 1988 Jan;40(1):62–63. doi: 10.1111/j.2042-7158.1988.tb05154.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Shoji N., Ohizumi Y. Gingerol, a novel cardiotonic agent, activates the Ca2+-pumping ATPase in skeletal and cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1987 Sep 18;903(1):96–102. doi: 10.1016/0005-2736(87)90159-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Martonosi A. N. Mechanisms of Ca2+ release from sarcoplasmic reticulum of skeletal muscle. Physiol Rev. 1984 Oct;64(4):1240–1320. doi: 10.1152/physrev.1984.64.4.1240. [DOI] [PubMed] [Google Scholar]
- McPherson P. S., Campbell K. P. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993 Jul 5;268(19):13765–13768. [PubMed] [Google Scholar]
- Nakamura Y., Kobayashi J., Gilmore J., Mascal M., Rinehart K. L., Jr, Nakamura H., Ohizumi Y. Bromo-eudistomin D, a novel inducer of calcium release from fragmented sarcoplasmic reticulum that causes contractions of skinned muscle fibers. J Biol Chem. 1986 Mar 25;261(9):4139–4142. [PubMed] [Google Scholar]
- Palade P. Drug-induced Ca2+ release from isolated sarcoplasmic reticulum. III. Block of Ca2+-induced Ca2+ release by organic polyamines. J Biol Chem. 1987 May 5;262(13):6149–6154. [PubMed] [Google Scholar]
- Pessah I. N., Stambuk R. A., Casida J. E. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987 Mar;31(3):232–238. [PubMed] [Google Scholar]
- Saito A., Inui M., Radermacher M., Frank J., Fleischer S. Ultrastructure of the calcium release channel of sarcoplasmic reticulum. J Cell Biol. 1988 Jul;107(1):211–219. doi: 10.1083/jcb.107.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F. Membrane charge movement and depolarization-contraction coupling. Annu Rev Physiol. 1981;43:507–517. doi: 10.1146/annurev.ph.43.030181.002451. [DOI] [PubMed] [Google Scholar]
- Seino A., Kobayashi M., Kobayashi J., Fang Y. I., Ishibashi M., Nakamura H., Momose K., Ohizumi Y. 9-methyl-7-bromoeudistomin D, a powerful radio-labelable Ca++ releaser having caffeine-like properties, acts on Ca(++)-induced Ca++ release channels of sarcoplasmic reticulum. J Pharmacol Exp Ther. 1991 Mar;256(3):861–867. [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia H. H., Kirby M. S., Lederer W. J., Coronado R. Scorpion toxins targeted against the sarcoplasmic reticulum Ca(2+)-release channel of skeletal and cardiac muscle. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12185–12189. doi: 10.1073/pnas.89.24.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Damiani E., Maurer A., Tu A. T. Interaction of myotoxin a with the Ca2+-ATPase of skeletal muscle sarcoplasmic reticulum. Arch Biochem Biophys. 1986 Apr;246(1):90–97. doi: 10.1016/0003-9861(86)90452-2. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Grassucci R., Frank J., Saito A., Inui M., Fleischer S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature. 1989 Mar 9;338(6211):167–170. doi: 10.1038/338167a0. [DOI] [PubMed] [Google Scholar]


