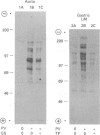
Abstract
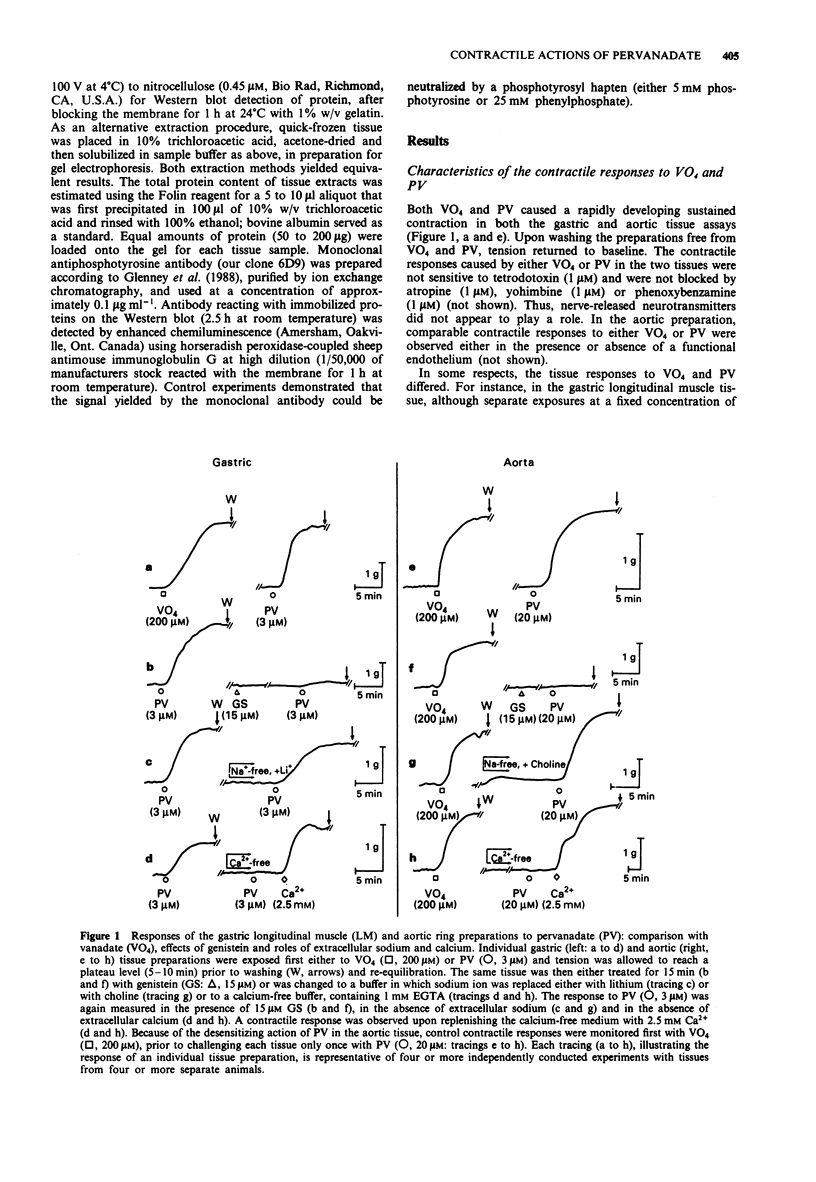
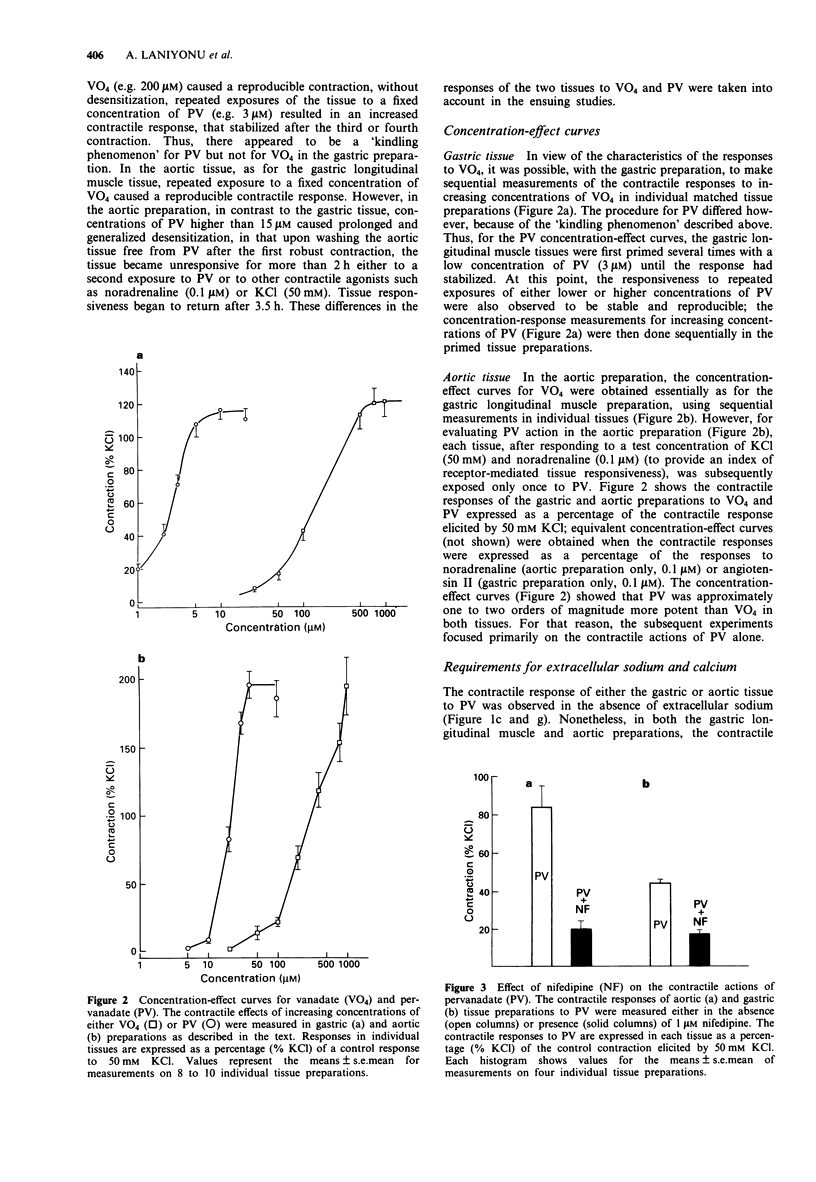
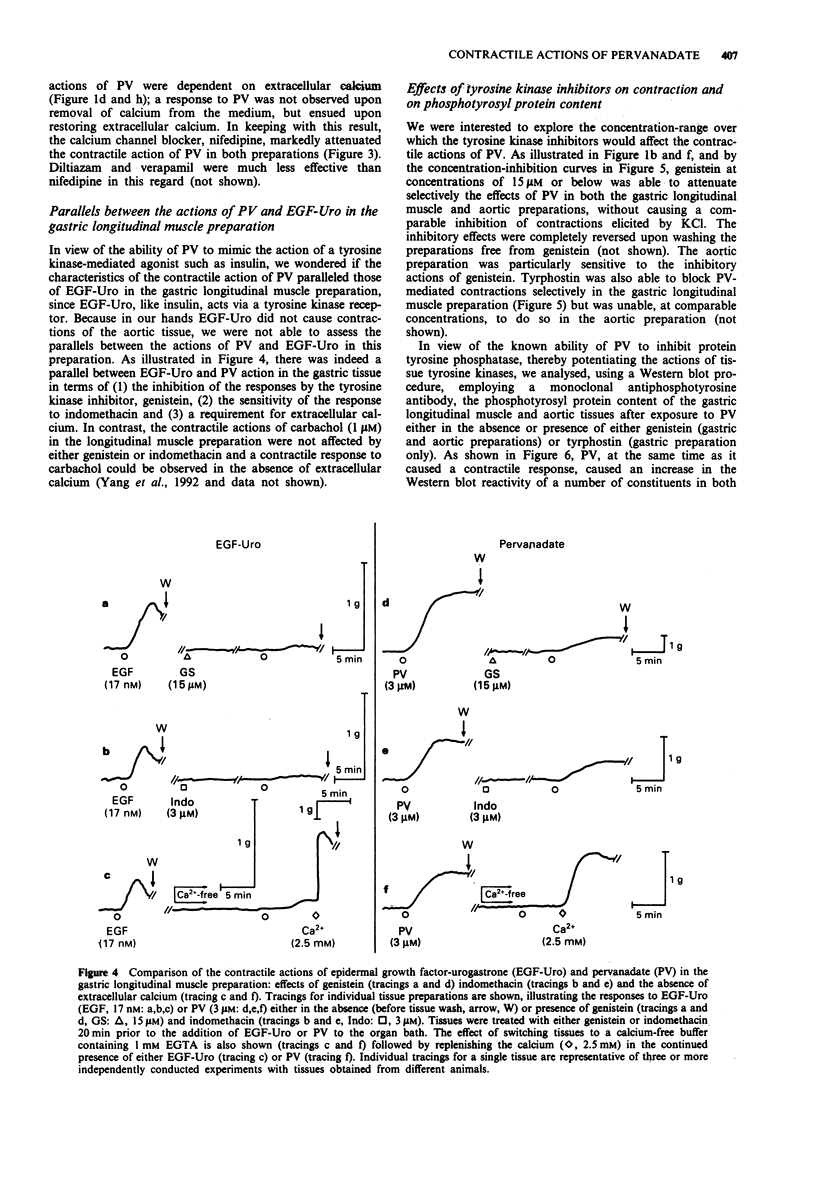
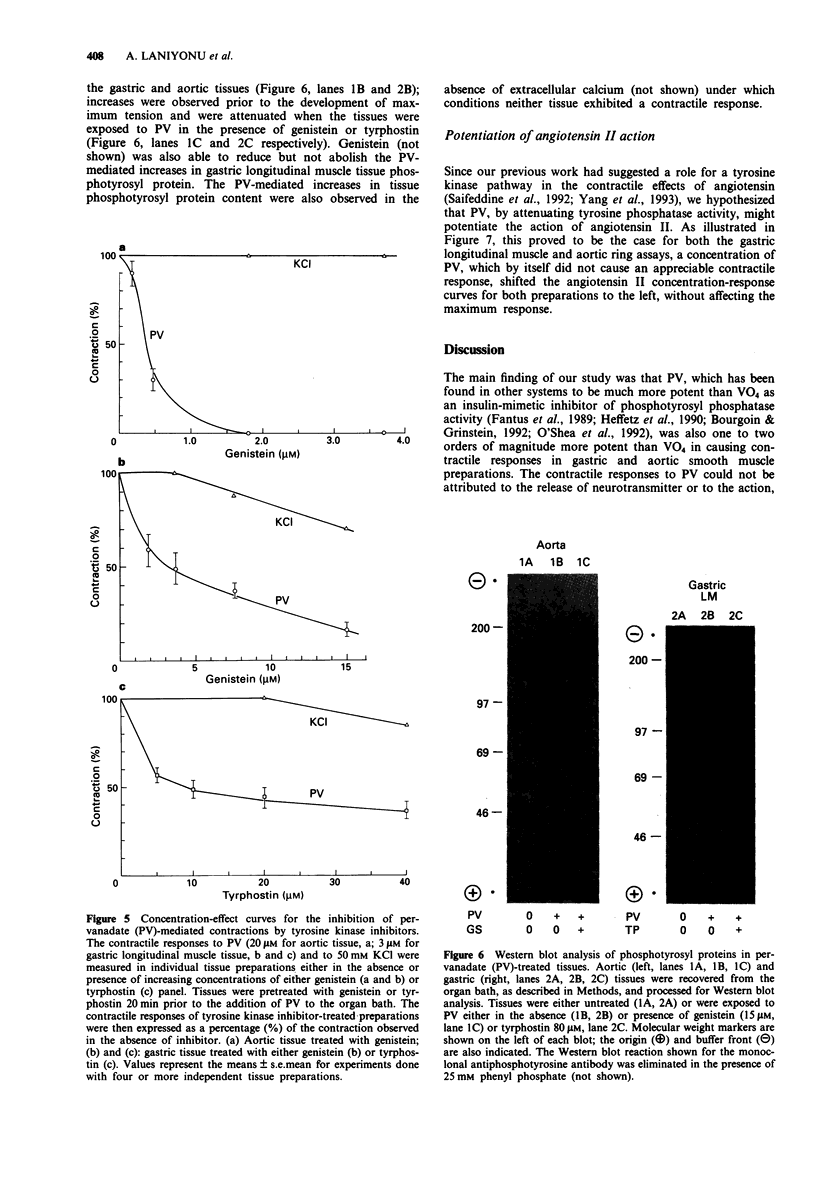
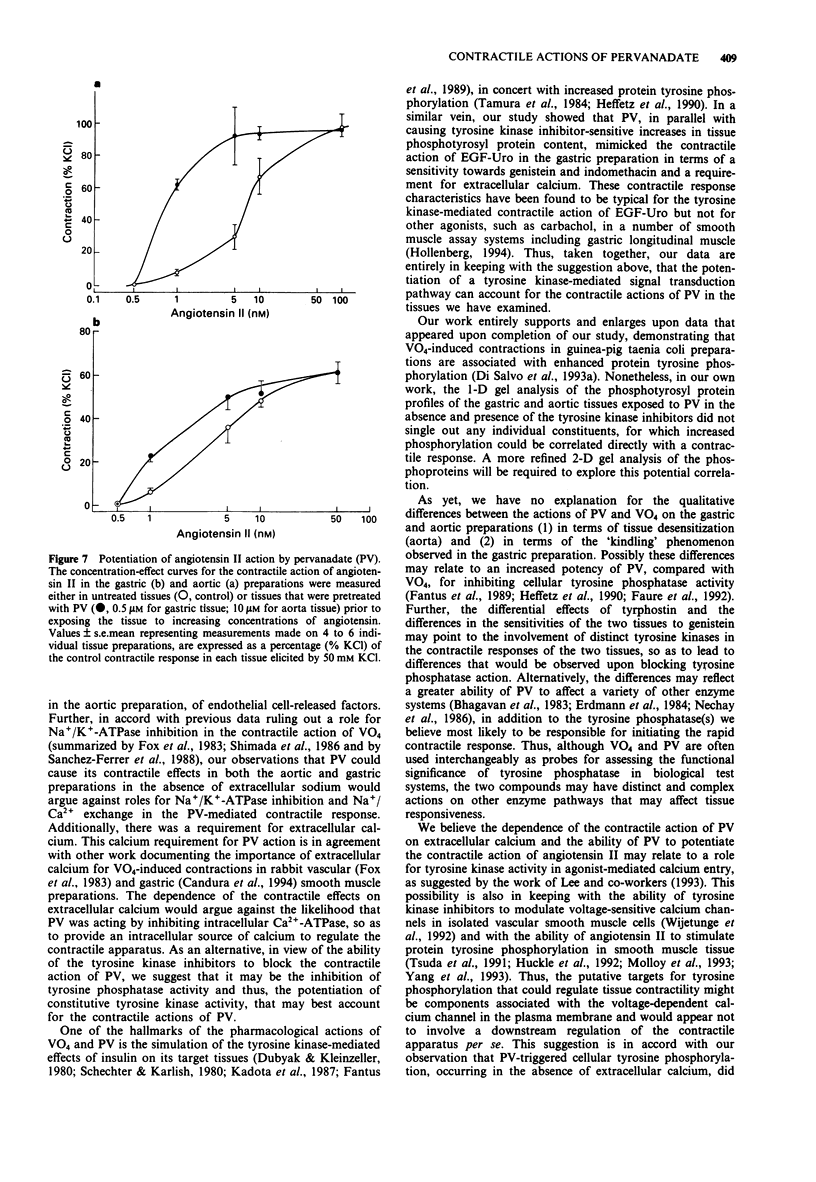
1. The contractile actions of vanadate (VO4) and pervanadate (PV, peroxide(s) of vanadate) were studied in rat gastric longitudinal muscle strips and in aortic rings. The roles of extracellular sodium and calcium were evaluated and the potential effects of nerve-released agonists were considered. The possibility that these responses were due to the potentiation of tyrosine kinase activity, as a result of PV-mediated tyrosine phosphatase inhibition was explored with the use of tyrosine kinase inhibitors (genistein, tyrphostin) and by Western blot analysis of phosphotyrosyl proteins in PV-treated tissues. The ability of PV to mimic the action of the tyrosine kinase receptor-associated agonist, epidermal growth factor-urogastrone (EGF-Uro), in the gastric preparation was also studied. 2. PV caused concentration-dependent contractions in both gastric and aorta-derived tissues, with a potency that was 1 to 2 orders of magnitude greater than that of VO4. 3. Although repeated exposure of gastric and aortic tissues to a fixed concentration of VO4 caused reproducible contractions in both tissues, repeated exposure of gastric tissue to PV caused an increased contractile response plateauing after 3 exposures. In contrast, a single exposure of aortic tissue to PV (20 microM) caused a prolonged desensitization of the tissue to the subsequent contractile actions of PV or other agonists. 4. The contractile responses to PV were unaffected in both preparations by tetrodotoxin, atropine, yohimbine and phenoxybenzamine; and in the aortic preparation, the responses to VO4 and PV were the same in the presence or absence of a functional endothelium.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk B. C., Alexander R. W. Vasoactive effects of growth factors. Biochem Pharmacol. 1989 Jan 15;38(2):219–225. doi: 10.1016/0006-2952(89)90030-0. [DOI] [PubMed] [Google Scholar]
- Blake R. A., Walker T. R., Watson S. P. Activation of human platelets by peroxovanadate is associated with tyrosine phosphorylation of phospholipase C gamma and formation of inositol phosphates. Biochem J. 1993 Mar 1;290(Pt 2):471–475. doi: 10.1042/bj2900471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgoin S., Grinstein S. Peroxides of vanadate induce activation of phospholipase D in HL-60 cells. Role of tyrosine phosphorylation. J Biol Chem. 1992 Jun 15;267(17):11908–11916. [PubMed] [Google Scholar]
- Candura S. M., Manzo L., Marraccini P., Coccini T., Tonini M. Investigation into vanadate-induced potentiation of smooth muscle contractility in the rabbit isolated ileum. Life Sci. 1994;54(4):237–244. doi: 10.1016/0024-3205(94)00812-4. [DOI] [PubMed] [Google Scholar]
- Di Salvo J., Semenchuk L. A., Lauer J. Vanadate-induced contraction of smooth muscle and enhanced protein tyrosine phosphorylation. Arch Biochem Biophys. 1993 Aug 1;304(2):386–391. doi: 10.1006/abbi.1993.1366. [DOI] [PubMed] [Google Scholar]
- Di Salvo J., Steusloff A., Semenchuk L., Satoh S., Kolquist K., Pfitzer G. Tyrosine kinase inhibitors suppress agonist-induced contraction in smooth muscle. Biochem Biophys Res Commun. 1993 Feb 15;190(3):968–974. doi: 10.1006/bbrc.1993.1144. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., Kleinzeller A. The insulin-mimetic effects of vanadate in isolated rat adipocytes. Dissociation from effects of vanadate as a (Na+-K+)ATPase inhibitor. J Biol Chem. 1980 Jun 10;255(11):5306–5312. [PubMed] [Google Scholar]
- Erdmann E., Werdan K., Krawietz W., Schmitz W., Scholz H. Vanadate and its significance in biochemistry and pharmacology. Biochem Pharmacol. 1984 Apr 1;33(7):945–950. doi: 10.1016/0006-2952(84)90498-2. [DOI] [PubMed] [Google Scholar]
- Fantus I. G., Kadota S., Deragon G., Foster B., Posner B. I. Pervanadate [peroxide(s) of vanadate] mimics insulin action in rat adipocytes via activation of the insulin receptor tyrosine kinase. Biochemistry. 1989 Oct 31;28(22):8864–8871. doi: 10.1021/bi00448a027. [DOI] [PubMed] [Google Scholar]
- Faure R., Baquiran G., Bergeron J. J., Posner B. I. The dephosphorylation of insulin and epidermal growth factor receptors. Role of endosome-associated phosphotyrosine phosphatase(s). J Biol Chem. 1992 Jun 5;267(16):11215–11221. [PubMed] [Google Scholar]
- Fox A. A., Borchard U., Neumann M. Effects of vanadate on isolated vascular tissue: biochemical and functional investigations. J Cardiovasc Pharmacol. 1983 Mar-Apr;5(2):309–316. doi: 10.1097/00005344-198303000-00024. [DOI] [PubMed] [Google Scholar]
- Gazit A., Yaish P., Gilon C., Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Furuya W., Lu D. J., Mills G. B. Vanadate stimulates oxygen consumption and tyrosine phosphorylation in electropermeabilized human neutrophils. J Biol Chem. 1990 Jan 5;265(1):318–327. [PubMed] [Google Scholar]
- Heffetz D., Bushkin I., Dror R., Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem. 1990 Feb 15;265(5):2896–2902. [PubMed] [Google Scholar]
- Hollenberg M. D., Laniyonu A. A., Saifeddine M., Moore G. J. Role of the amino- and carboxyl-terminal domains of thrombin receptor-derived polypeptides in biological activity in vascular endothelium and gastric smooth muscle: evidence for receptor subtypes. Mol Pharmacol. 1993 Jun;43(6):921–930. [PubMed] [Google Scholar]
- Hollenberg M. D. The acute actions of growth factors in smooth muscle systems. Life Sci. 1994;54(4):223–235. doi: 10.1016/0024-3205(94)00811-6. [DOI] [PubMed] [Google Scholar]
- Huckle W. R., Dy R. C., Earp H. S. Calcium-dependent increase in tyrosine kinase activity stimulated by angiotensin II. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8837–8841. doi: 10.1073/pnas.89.18.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandhyala B. S., Hom G. J. Minireview: physiological and pharmacological properties of vanadium. Life Sci. 1983 Oct 3;33(14):1325–1340. doi: 10.1016/0024-3205(83)90816-0. [DOI] [PubMed] [Google Scholar]
- Kadota S., Fantus I. G., Deragon G., Guyda H. J., Hersh B., Posner B. I. Peroxide(s) of vanadium: a novel and potent insulin-mimetic agent which activates the insulin receptor kinase. Biochem Biophys Res Commun. 1987 Aug 31;147(1):259–266. doi: 10.1016/s0006-291x(87)80115-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. M., Toscas K., Villereal M. L. Inhibition of bradykinin- and thapsigargin-induced Ca2+ entry by tyrosine kinase inhibitors. J Biol Chem. 1993 May 15;268(14):9945–9948. [PubMed] [Google Scholar]
- Molloy C. J., Taylor D. S., Weber H. Angiotensin II stimulation of rapid protein tyrosine phosphorylation and protein kinase activation in rat aortic smooth muscle cells. J Biol Chem. 1993 Apr 5;268(10):7338–7345. [PubMed] [Google Scholar]
- O'Shea J. J., McVicar D. W., Bailey T. L., Burns C., Smyth M. J. Activation of human peripheral blood T lymphocytes by pharmacological induction of protein-tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10306–10310. doi: 10.1073/pnas.89.21.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Urakawa N. Effects of vanadate on mechanical responses and Na-K pump in vascular smooth muscle. Eur J Pharmacol. 1980 Dec 5;68(3):339–347. doi: 10.1016/0014-2999(80)90531-2. [DOI] [PubMed] [Google Scholar]
- Role of vanadium in biology. Symposium summary. Fed Proc. 1986 Feb;45(2):123–132. [PubMed] [Google Scholar]
- Shechter Y., Karlish S. J. Insulin-like stimulation of glucose oxidation in rat adipocytes by vanadyl (IV) ions. Nature. 1980 Apr 10;284(5756):556–558. doi: 10.1038/284556a0. [DOI] [PubMed] [Google Scholar]
- Shimada T., Shimamura K., Sunano S. Effects of sodium vanadate on various types of vascular smooth muscles. Blood Vessels. 1986;23(3):113–124. doi: 10.1159/000158628. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ferrer C. F., Marín J., Lluch M., Valverde A., Salaices M. Actions of vanadate on vascular tension and sodium pump activity in cat isolated cerebral and femoral arteries. Br J Pharmacol. 1988 Jan;93(1):53–60. doi: 10.1111/j.1476-5381.1988.tb11404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S., Brown T. A., Whipple J. H., Fujita-Yamaguchi Y., Dubler R. E., Cheng K., Larner J. A novel mechanism for the insulin-like effect of vanadate on glycogen synthase in rat adipocytes. J Biol Chem. 1984 May 25;259(10):6650–6658. [PubMed] [Google Scholar]
- Tsuda T., Kawahara Y., Shii K., Koide M., Ishida Y., Yokoyama M. Vasoconstrictor-induced protein-tyrosine phosphorylation in cultured vascular smooth muscle cells. FEBS Lett. 1991 Jul 8;285(1):44–48. doi: 10.1016/0014-5793(91)80721-e. [DOI] [PubMed] [Google Scholar]
- Wijetunge S., Aalkjaer C., Schachter M., Hughes A. D. Tyrosine kinase inhibitors block calcium channel currents in vascular smooth muscle cells. Biochem Biophys Res Commun. 1992 Dec 30;189(3):1620–1623. doi: 10.1016/0006-291x(92)90262-j. [DOI] [PubMed] [Google Scholar]
- Yang S. G., Saifeddine M., Hollenberg M. D. Tyrosine kinase inhibitors and the contractile action of epidermal growth factor-urogastrone and other agonists in gastric smooth muscle. Can J Physiol Pharmacol. 1992 Jan;70(1):85–93. doi: 10.1139/y92-012. [DOI] [PubMed] [Google Scholar]
- Yang S. G., Saifeddine M., Laniyonu A., Hollenberg M. D. Distinct signal transduction pathways for angiotensin-II in guinea pig gastric smooth muscle: differential blockade by indomethacin and tyrosine kinase inhibitors. J Pharmacol Exp Ther. 1993 Feb;264(2):958–966. [PubMed] [Google Scholar]