Abstract
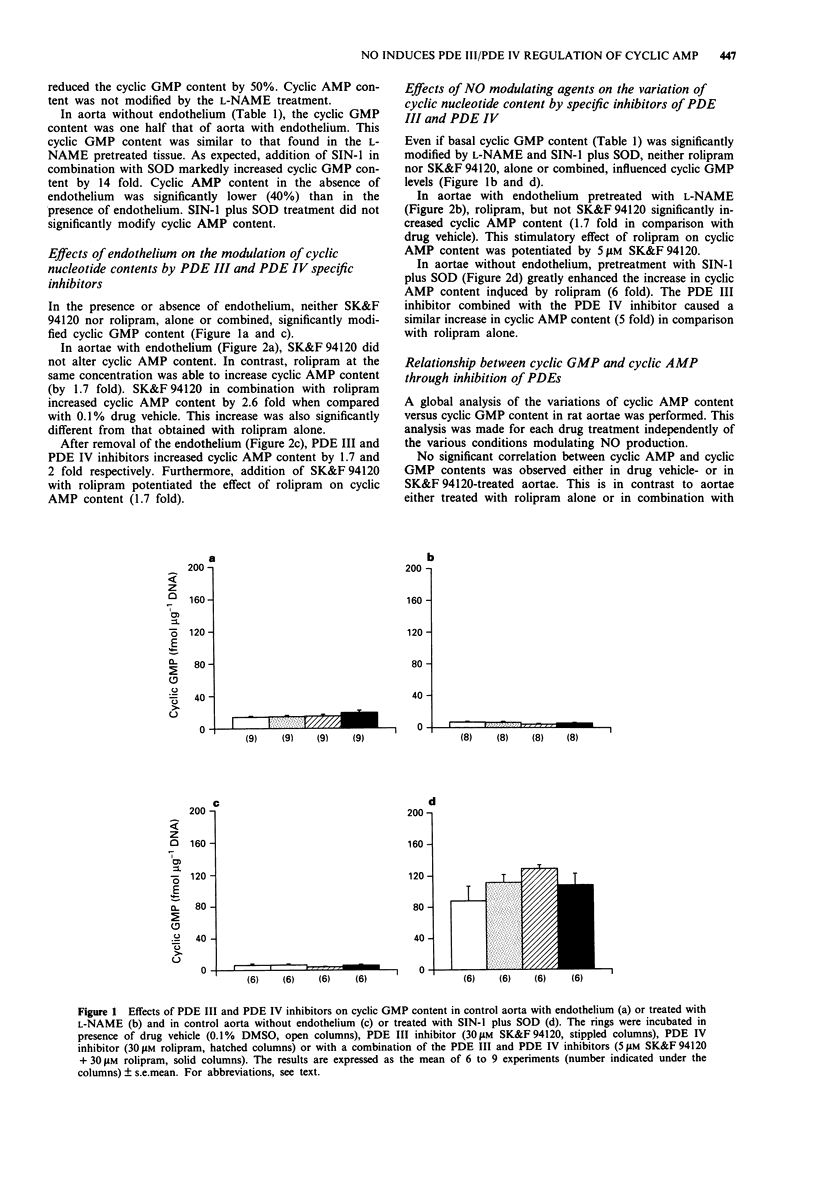
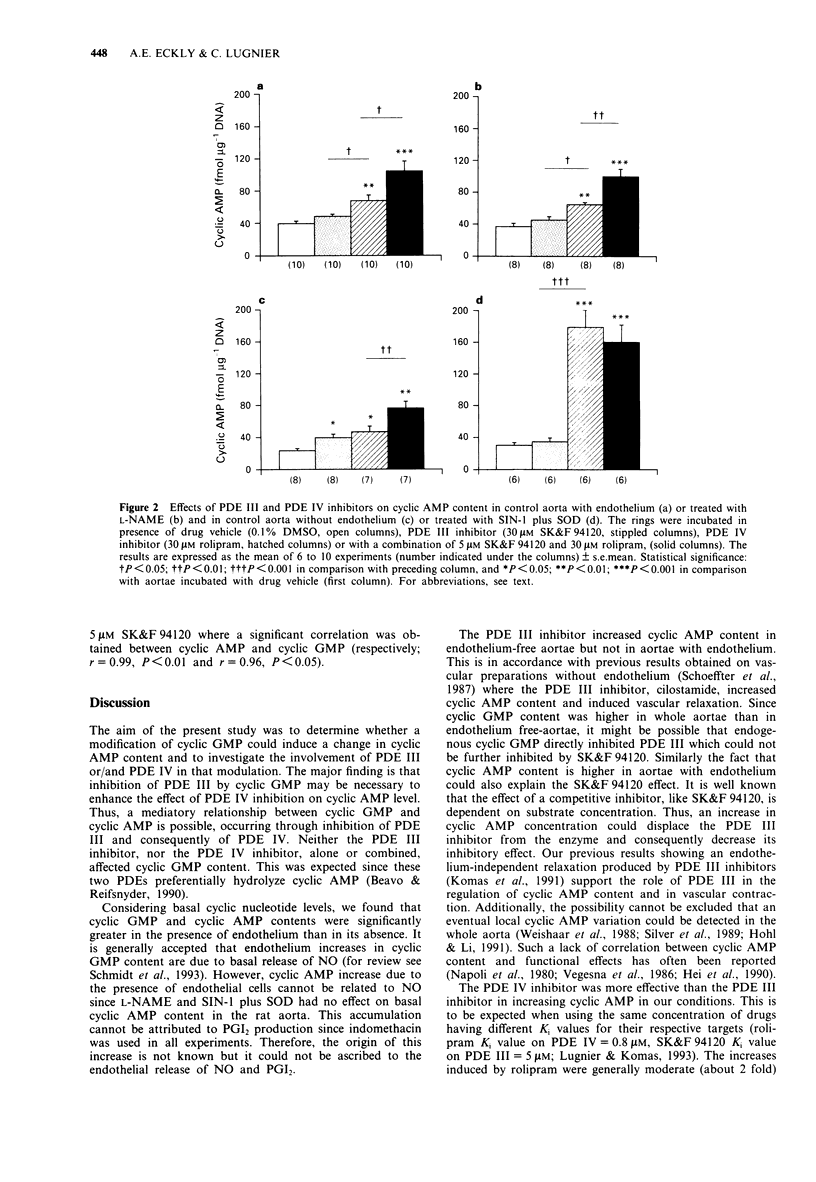
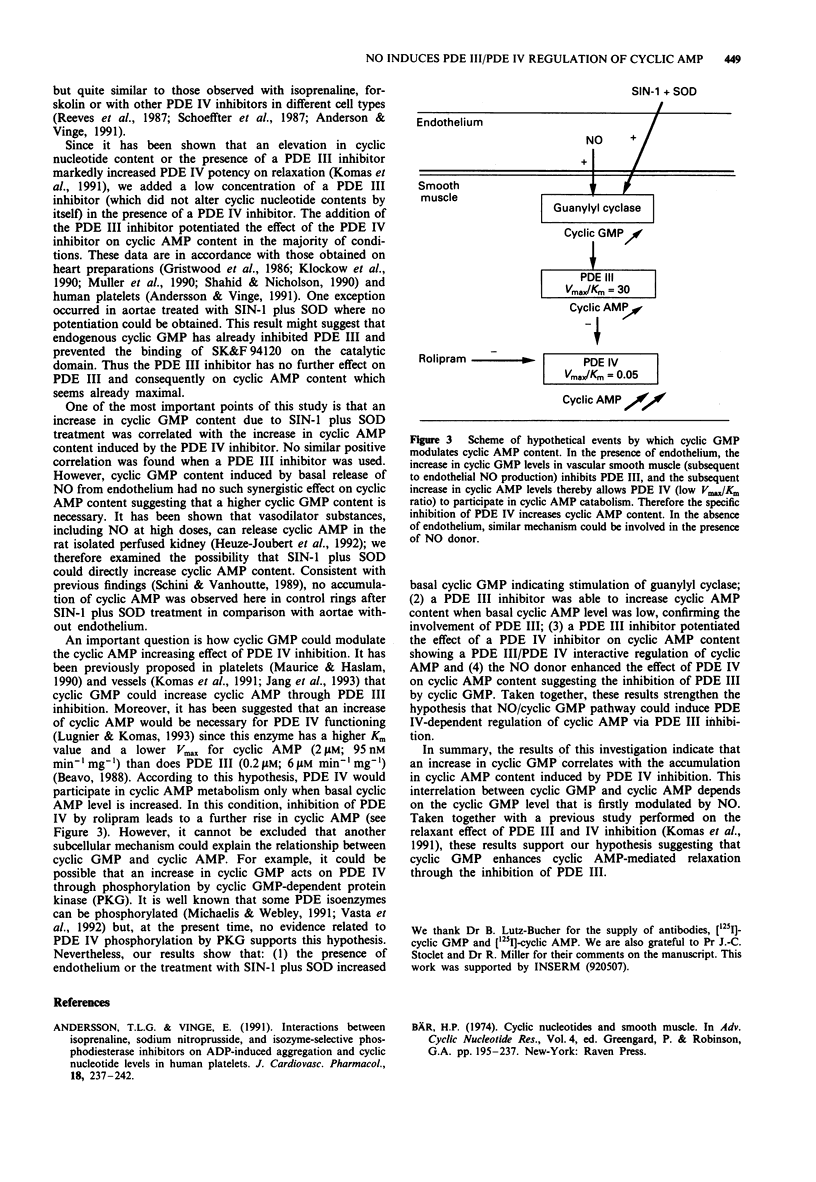
1. The effect on cyclic nucleotide contents of selective inhibitors of cyclic nucleotide phosphodiesterase (PDE) isoforms III and IV (respectively SK&F 94120 and rolipram) and their interactions with endothelium and NO have been studied in rat aorta in the presence of indomethacin (10 microM). The participation of NO was assessed by using either NG-nitro-L-arginine methyl ester (L-NAME) (NO synthase inhibitor: 30 microM) or 3-morpholinosydnonimine (SIN-1, NO donor: 10 microM with SOD 100 units ml-1). 2. The presence of endothelium significantly increased both adenosine 3':5'-cyclic monophosphate (cyclic AMP, 1.7 fold) and guanosine 3':5'-cyclic monophosphate (cyclic GMP, 2.2 fold) contents. Cyclic GMP was largely affected by L-NAME or SIN-1 treatment, this was not the case for cyclic AMP suggesting that the presence of endothelium modified cyclic AMP content in aorta independently of the NO production. 3. In the presence or absence of endothelium, neither SK&F 94120 nor rolipram, alone or combined, significantly modified cyclic GMP content. 4. The PDE III inhibitor significantly affected cyclic AMP content only in non treated aorta without endothelium. In contrast, the PDE IV inhibitor increased cyclic AMP in all conditions. These increases were generally about 2 fold but markedly higher in aorta treated with SIN-1 and superoxide dismutase (SOD, 6 fold). Association of a low concentration of the PDE III inhibitor (5 microM) with the PDE IV inhibitor (30 microM) potentiated the effect of the PDE IV inhibitor on cyclic AMP content, except for aorta without endothelium treated with SIN-1 plus SOD.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T. L., Vinge E. Interactions between isoprenaline, sodium nitroprusside, and isozyme-selective phosphodiesterase inhibitors on ADP-induced aggregation and cyclic nucleotide levels in human platelets. J Cardiovasc Pharmacol. 1991 Aug;18(2):237–242. doi: 10.1097/00005344-199108000-00010. [DOI] [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Bär H. P. Cyclic nucleotides and smooth muscle. Adv Cyclic Nucleotide Res. 1974;4(0):195–237. [PubMed] [Google Scholar]
- Cailla H. L., Racine-Weisbuch M. S., Delaage M. A. Adenosine 3',5' cyclic monophosphate assay at 10-15 mole level. Anal Biochem. 1973 Dec;56(2):394–407. doi: 10.1016/0003-2697(73)90205-4. [DOI] [PubMed] [Google Scholar]
- Cailla H. L., Vannier C. J., Delaage M. A. Guanosine 3', 5'-cyclicmonophosphate assay at 10(-15)-mole level. Anal Biochem. 1976 Jan;70(1):195–202. doi: 10.1016/s0378-5173(83)90100-x. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Henderson A. H., Edwards D. H., Lewis M. J. Isolated perfused rabbit coronary artery and aortic strip preparations: the role of endothelium-derived relaxant factor. J Physiol. 1984 Jun;351:13–24. doi: 10.1113/jphysiol.1984.sp015228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hei Y. J., MacDonell K. L., McNeill J. H., Diamond J. Lack of correlation between activation of cyclic AMP-dependent protein kinase and inhibition of contraction of rat vas deferens by cyclic AMP analogs. Mol Pharmacol. 1991 Feb;39(2):233–238. [PubMed] [Google Scholar]
- Heuzé-Joubert I., Mennecier P., Simonet S., Laubie M., Verbeuren T. J. Effect of vasodilators, including nitric oxide, on the release of cGMP and cAMP in the isolated perfused rat kidney. Eur J Pharmacol. 1992 Sep 22;220(2-3):161–171. doi: 10.1016/0014-2999(92)90744-o. [DOI] [PubMed] [Google Scholar]
- Hohl C. M., Li Q. A. Compartmentation of cAMP in adult canine ventricular myocytes. Relation to single-cell free Ca2+ transients. Circ Res. 1991 Nov;69(5):1369–1379. doi: 10.1161/01.res.69.5.1369. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Harbison R. G., Wood K. S., Kadowitz P. J. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986 Jun;237(3):893–900. [PubMed] [Google Scholar]
- Jang E. K., Davidson M. M., Crankshaw D., Haslam R. J. Synergistic inhibitory effects of atriopeptin II and isoproterenol on contraction of rat aortic smooth muscle: roles of cGMP and cAMP. Eur J Pharmacol. 1993 Dec 21;250(3):477–481. doi: 10.1016/0014-2999(93)90038-j. [DOI] [PubMed] [Google Scholar]
- Komas N., Lugnier C., Stoclet J. C. Endothelium-dependent and independent relaxation of the rat aorta by cyclic nucleotide phosphodiesterase inhibitors. Br J Pharmacol. 1991 Oct;104(2):495–503. doi: 10.1111/j.1476-5381.1991.tb12457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukovetz W. R., Graier W. F., Groschner K. Contribution of agonist-induced hyperpolarization to Ca2+ influx and formation of EDRF in vascular endothelial cells. Jpn J Pharmacol. 1992;58 (Suppl 2):213P–219P. [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L. Towards an understanding of the mechanism of action of cyclic AMP and cyclic GMP in smooth muscle relaxation. Blood Vessels. 1991;28(1-3):129–137. doi: 10.1159/000158852. [DOI] [PubMed] [Google Scholar]
- Lindgren S., Rascón A., Andersson K. E., Manganiello V., Degerman E. Selective inhibition of cGMP-inhibited and cGMP-noninhibited cyclic nucleotide phosphodiesterases and relaxation of rat aorta. Biochem Pharmacol. 1991 Jul 15;42(3):545–552. doi: 10.1016/0006-2952(91)90317-x. [DOI] [PubMed] [Google Scholar]
- Lugnier C., Komas N. Modulation of vascular cyclic nucleotide phosphodiesterases by cyclic GMP: role in vasodilatation. Eur Heart J. 1993 Nov;14 (Suppl 1):141–148. [PubMed] [Google Scholar]
- Lugnier C., Schini V. B. Characterization of cyclic nucleotide phosphodiesterases from cultured bovine aortic endothelial cells. Biochem Pharmacol. 1990 Jan 1;39(1):75–84. doi: 10.1016/0006-2952(90)90650-a. [DOI] [PubMed] [Google Scholar]
- Lugnier C., Schoeffter P., Le Bec A., Strouthou E., Stoclet J. C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986 May 15;35(10):1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Maurice D. H., Crankshaw D., Haslam R. J. Synergistic actions of nitrovasodilators and isoprenaline on rat aortic smooth muscle. Eur J Pharmacol. 1991 Jan 10;192(2):235–242. doi: 10.1016/0014-2999(91)90048-u. [DOI] [PubMed] [Google Scholar]
- Maurice D. H., Haslam R. J. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol. 1990 May;37(5):671–681. [PubMed] [Google Scholar]
- Michael A. E., Webley G. E. Prostaglandin F2 alpha stimulates cAMP phosphodiesterase via protein kinase C in cultured human granulosa cells. Mol Cell Endocrinol. 1991 Dec;82(2-3):207–214. doi: 10.1016/0303-7207(91)90033-o. [DOI] [PubMed] [Google Scholar]
- Muller B., Lugnier C., Stoclet J. C. Involvement of rolipram-sensitive cyclic AMP phosphodiesterase in the regulation of cardiac contraction. J Cardiovasc Pharmacol. 1990 Nov;16(5):796–803. doi: 10.1097/00005344-199011000-00016. [DOI] [PubMed] [Google Scholar]
- Muller B., Stoclet J. C., Lugnier C. Cytosolic and membrane-bound cyclic nucleotide phosphodiesterases from guinea pig cardiac ventricles. Eur J Pharmacol. 1992 Mar 12;225(3):263–272. doi: 10.1016/0922-4106(92)90028-t. [DOI] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli S. A., Gruetter C. A., Ignarro L. J., Kadowitz P. J. Relaxation of bovine coronary arterial smooth muscle by cyclic GMP, cyclic AMP and analogs. J Pharmacol Exp Ther. 1980 Mar;212(3):469–473. [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Schimmer B. P., Wong M. Genetics of ACTH action in Y1 mouse adrenocortical tumor cells. Proc West Pharmacol Soc. 1986;29:303–305. [PubMed] [Google Scholar]
- Schini V. B., Vanhoutte P. M. SIN-1 stimulates the production of cyclic GMP but not cyclic AMP in porcine aortic endothelial cells. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S91–S94. [PubMed] [Google Scholar]
- Schmidt H. H., Lohmann S. M., Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993 Aug 18;1178(2):153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Lugnier C., Demesy-Waeldele F., Stoclet J. C. Role of cyclic AMP- and cyclic GMP-phosphodiesterases in the control of cyclic nucleotide levels and smooth muscle tone in rat isolated aorta. A study with selective inhibitors. Biochem Pharmacol. 1987 Nov 15;36(22):3965–3972. doi: 10.1016/0006-2952(87)90465-5. [DOI] [PubMed] [Google Scholar]
- Setaro F., Morley C. G. A modified fluorometric method for the determination of microgram quantities of DNA from cell or tissue cultures. Anal Biochem. 1976 Mar;71(1):313–317. doi: 10.1016/0003-2697(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Shahid M., Nicholson C. D. Comparison of cyclic nucleotide phosphodiesterase isoenzymes in rat and rabbit ventricular myocardium: positive inotropic and phosphodiesterase inhibitory effects of Org 30029, milrinone and rolipram. Naunyn Schmiedebergs Arch Pharmacol. 1990 Dec;342(6):698–705. doi: 10.1007/BF00175715. [DOI] [PubMed] [Google Scholar]
- Silver P. J., Lepore R. E., O'Connor B., Lemp B. M., Hamel L. T., Bentley R. G., Harris A. L. Inhibition of the low Km cyclic AMP phosphodiesterase and activation of the cyclic AMP system in vascular smooth muscle by milrinone. J Pharmacol Exp Ther. 1988 Oct;247(1):34–42. [PubMed] [Google Scholar]
- Souness J. E., Diocee B. K., Martin W., Moodie S. A. Pig aortic endothelial-cell cyclic nucleotide phosphodiesterases. Use of phosphodiesterase inhibitors to evaluate their roles in regulating cyclic nucleotide levels in intact cells. Biochem J. 1990 Feb 15;266(1):127–132. doi: 10.1042/bj2660127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta V., Smith C. J., Calvo J., Belfrage P., Manganiello V. C. Insulin and isoproterenol induce phosphorylation of the particulate cyclic GMP-inhibited, low Km cyclic AMP phosphodiesterase (cGI PDE) in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1992 Mar 31;183(3):1070–1075. doi: 10.1016/s0006-291x(05)80299-2. [DOI] [PubMed] [Google Scholar]


