Abstract
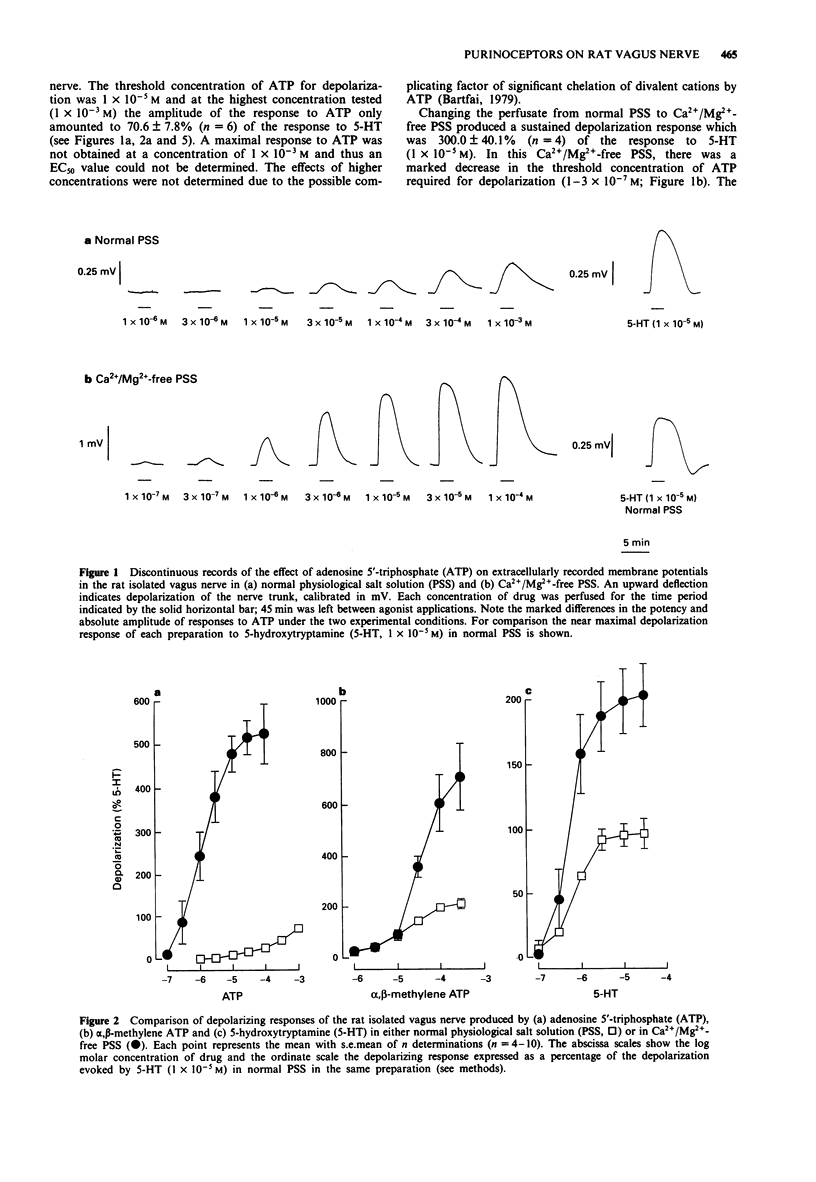
1. By use of a 'grease-gap' technique, the depolarizing effects of adenosine 5'-triphosphate (ATP) and ATP analogues on the rat isolated vagus nerve were determined in normal and in Ca2+/Mg(2+)-free (+ 1 x 10(-3) M ethylenediamine tetraacetic acid) physiological salt solution (PSS). 2. In normal PSS, ATP produced concentration-dependent depolarization responses but the concentration-effect curve to ATP was incomplete and a maximum effect was not achieved. The threshold concentration for depolarization was 1 x 10(-5) M and at the highest concentration tested (1 x 10(-3) M) the peak amplitude of the response to ATP only amounted to 71% of the depolarization produced by a near maximal response to 5-hydroxytryptamine (5-HT, 1 x 10(-5) M). 3. In Ca2+/Mg(2+)-free PSS, ATP produced depolarization responses at much lower concentrations and of markedly larger amplitude. Under these conditions, the threshold concentration for depolarization was 1-3 x 10(-7) M and the maximal response to ATP amounted to 526% of the response to 5-HT (1 x 10(-5) M) in normal PSS. The concentration-effect curve to ATP was sigmoid, with a defined maximum effect and a mean EC50 value of 1.2 x 10(-6) M. 4. In contrast to the effects on responses to ATP, the absence of divalent cations in the PSS did not modify the effective concentrations of either alpha, beta-methylene ATP or 5-HT. However, the maximum responses to both alpha, beta-methylene ATP and 5-HT were significantly increased in Ca2+/Mg(2+)-free PSS.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

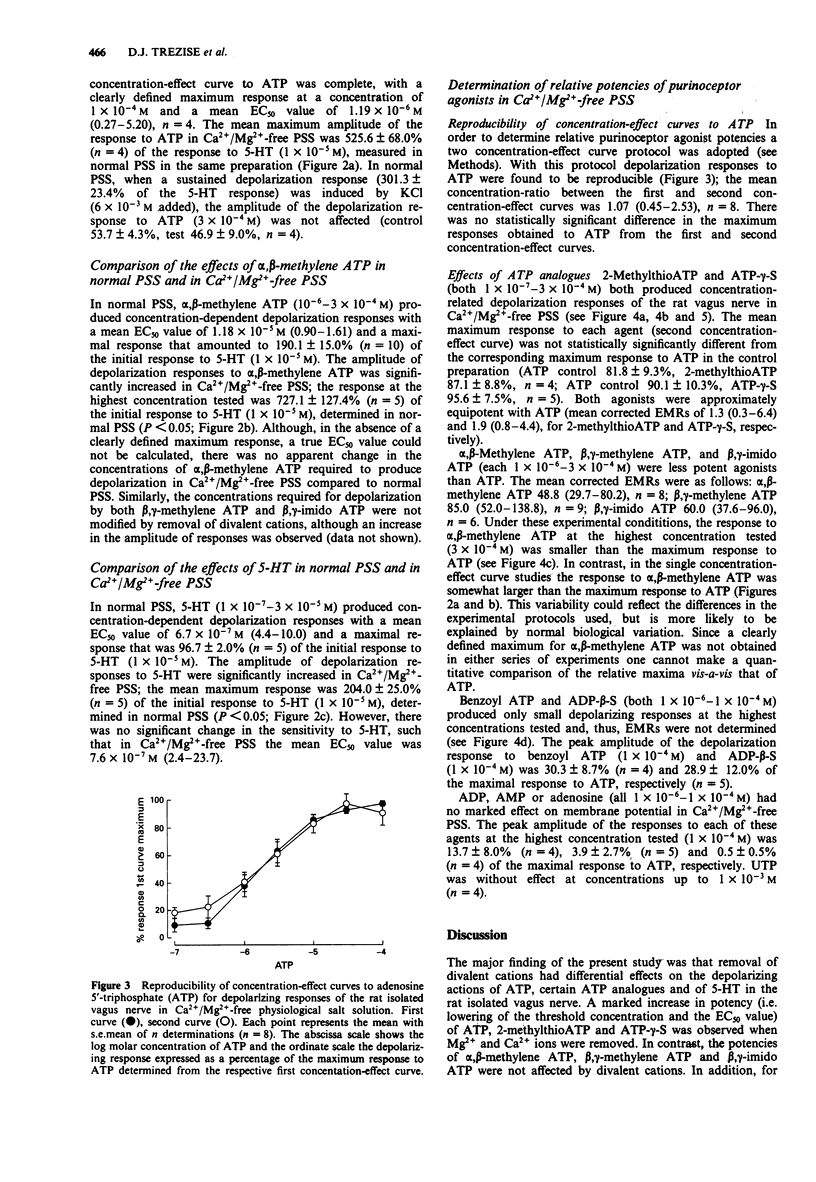
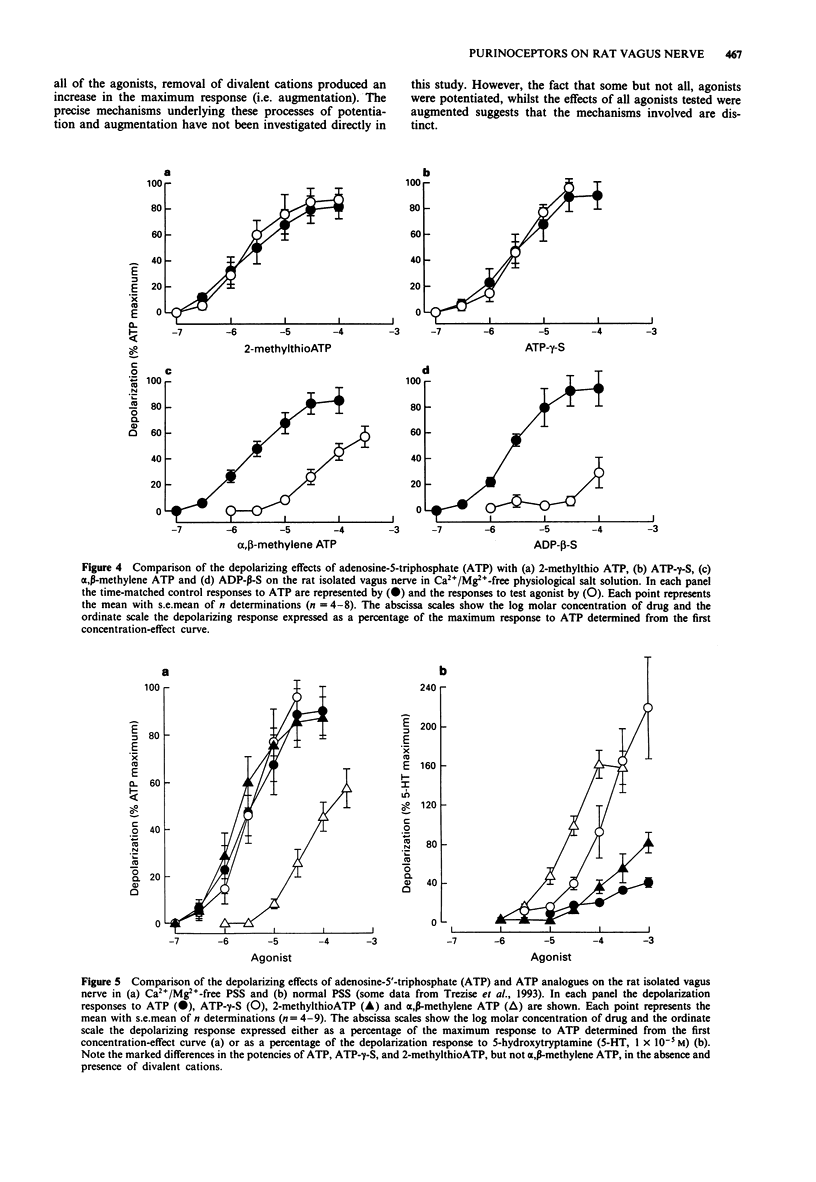



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. G., Burnstock G. The actions of adenosine 5'-triphosphate on guinea-pig intracardiac neurones in culture. Br J Pharmacol. 1990 Jun;100(2):269–276. doi: 10.1111/j.1476-5381.1990.tb15794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Berrie C. P., Hawkins P. T., Stephens L. R., Harden T. K., Downes C. P. Phosphatidylinositol 4,5-bisphosphate hydrolysis in turkey erythrocytes is regulated by P2y purinoceptors. Mol Pharmacol. 1989 Apr;35(4):526–532. [PubMed] [Google Scholar]
- Boyer J. L., Downes C. P., Harden T. K. Kinetics of activation of phospholipase C by P2Y purinergic receptor agonists and guanine nucleotides. J Biol Chem. 1989 Jan 15;264(2):884–890. [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Cascalheira J. F., Sebastião A. M. Adenine nucleotide analogues, including gamma-phosphate-substituted analogues, are metabolised extracellularly in innervated frog sartorius muscle. Eur J Pharmacol. 1992 Nov 3;222(1):49–59. doi: 10.1016/0014-2999(92)90462-d. [DOI] [PubMed] [Google Scholar]
- Cloues R., Jones S., Brown D. A. Zn2+ potentiates ATP-activated currents in rat sympathetic neurons. Pflugers Arch. 1993 Jul;424(2):152–158. doi: 10.1007/BF00374606. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. The ATP4- receptor of rat mast cells. Biochem J. 1980 Jun 15;188(3):789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack N. J., Hourani S. M. Some pharmacological and biochemical interactions of the enantiomers of adenylyl 5'-(beta, gamma-methylene)-diphosphonate with the guinea-pig urinary bladder. Br J Pharmacol. 1984 May;82(1):155–159. doi: 10.1111/j.1476-5381.1984.tb16453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack N. J., Pearson J. D., Gordon J. L. Stereoselectivity of ectonucleotidases on vascular endothelial cells. Biochem J. 1983 Sep 15;214(3):975–981. doi: 10.1042/bj2140975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Adenosine triphosphate-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:239–256. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J., Cole P., Davidson J. S. Extracellular nucleotides stimulate receptor-mediated calcium mobilization and inositol phosphate production in human fibroblasts. Biochem J. 1989 Oct 15;263(2):371–376. doi: 10.1042/bj2630371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol. 1988 Jul;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourani S. M., Welford L. A., Cusack N. J. L-AMP-PCP, an ATP receptor agonist in guinea-pig bladder, is inactive on taenia coli. Eur J Pharmacol. 1985 Jan 22;108(2):197–200. doi: 10.1016/0014-2999(85)90726-5. [DOI] [PubMed] [Google Scholar]
- Inoue R., Brading A. F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990 Jul;100(3):619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C. P1- and P2-purinoceptor subtypes--an update. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:30–50. [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Obukhov A. G. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988 Dec;27(3):995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Obukhov A. G., Volkova T. M. Receptors for ATP in rat sensory neurones: the structure-function relationship for ligands. Br J Pharmacol. 1988 Dec;95(4):1057–1062. doi: 10.1111/j.1476-5381.1988.tb11739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig K. D., Shiau A. K., Brake A. J., Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig K. D., Sportiello M. G., Erb L., Weisman G. A. A nucleotide receptor in vascular endothelial cells is specifically activated by the fully ionized forms of ATP and UTP. Biochem J. 1992 Jun 15;284(Pt 3):733–739. doi: 10.1042/bj2840733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Cusack N. J., Carleton J. S., Gordon J. L. Specificity of P2-purinoceptor that mediates endothelium-dependent relaxation of the pig aorta. Eur J Pharmacol. 1985 Feb 5;108(3):295–299. doi: 10.1016/0014-2999(85)90452-2. [DOI] [PubMed] [Google Scholar]
- Motte S., Pirotton S., Boeynaems J. M. Evidence that a form of ATP uncomplexed with divalent cations is the ligand of P2y and nucleotide/P2u receptors on aortic endothelial cells. Br J Pharmacol. 1993 Aug;109(4):967–971. doi: 10.1111/j.1476-5381.1993.tb13715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash H. L., Wallis D. I. Effects of divalent cations on responses of a sympathetic ganglion to 5-hydroxytryptamine and 1,1-dimethyl-4-phenyl piperazinium. Br J Pharmacol. 1981 Jul;73(3):759–772. doi: 10.1111/j.1476-5381.1981.tb16813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor S. E., Dainty I. A., Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci. 1991 Apr;12(4):137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- O'Connor S. E., Wood B. E., Leff P. Characterization of P2x-receptors in rabbit isolated ear artery. Br J Pharmacol. 1990 Nov;101(3):640–644. doi: 10.1111/j.1476-5381.1990.tb14133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. A., Malone H. M., Lambert J. J. An electrophysiological investigation of the properties of 5-HT3 receptors of rabbit nodose ganglion neurones in culture. Br J Pharmacol. 1993 Oct;110(2):665–676. doi: 10.1111/j.1476-5381.1993.tb13863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Bevan S. Properties of 5-hydroxytryptamine3 receptor-gated currents in adult rat dorsal root ganglion neurones. Br J Pharmacol. 1991 Jan;102(1):272–276. doi: 10.1111/j.1476-5381.1991.tb12165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham P. E., Cusack N. J., Gomperts B. D. Characterisation of the ATP4- receptor that mediates permeabilisation of rat mast cells. Eur J Pharmacol. 1988 Feb 16;147(1):13–21. doi: 10.1016/0014-2999(88)90628-0. [DOI] [PubMed] [Google Scholar]
- Trezise D. J., Kennedy I., Humphrey P. P. Characterization of purinoceptors mediating depolarization of rat isolated vagus nerve. Br J Pharmacol. 1993 Nov;110(3):1055–1060. doi: 10.1111/j.1476-5381.1993.tb13920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S., Harata N., Inoue K., Akaike N. ATP-gated current in dissociated rat nucleus solitarii neurons. J Neurophysiol. 1992 Sep;68(3):778–785. doi: 10.1152/jn.1992.68.3.778. [DOI] [PubMed] [Google Scholar]
- Webb T. E., Simon J., Krishek B. J., Bateson A. N., Smart T. G., King B. F., Burnstock G., Barnard E. A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993 Jun 14;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Welford L. A., Cusack N. J., Hourani S. M. ATP analogues and the guinea-pig taenia coli: a comparison of the structure-activity relationships of ectonucleotidases with those of the P2-purinoceptor. Eur J Pharmacol. 1986 Oct 7;129(3):217–224. doi: 10.1016/0014-2999(86)90431-0. [DOI] [PubMed] [Google Scholar]
- Welford L. A., Cusack N. J., Hourani S. M. The structure-activity relationships of ectonucleotidases and of excitatory P2-purinoceptors: evidence that dephosphorylation of ATP analogues reduces pharmacological potency. Eur J Pharmacol. 1987 Sep 2;141(1):123–130. doi: 10.1016/0014-2999(87)90418-3. [DOI] [PubMed] [Google Scholar]


