Abstract
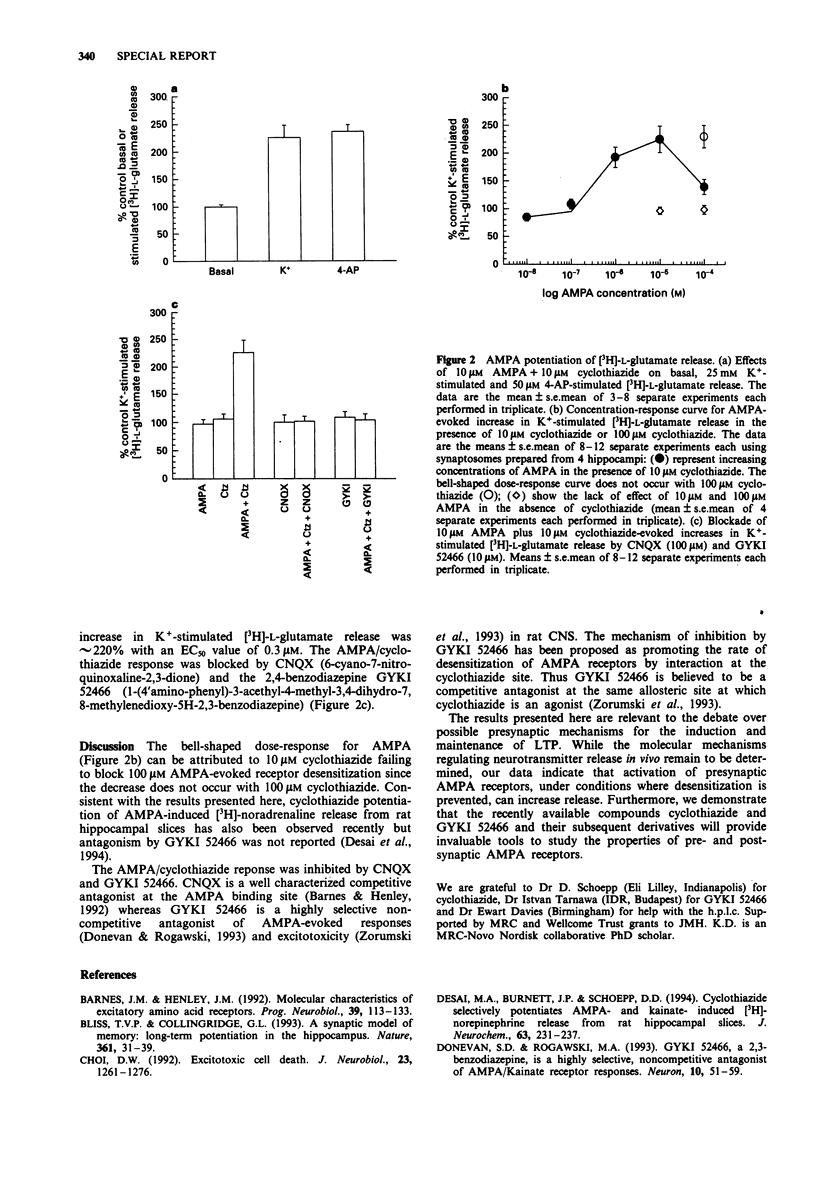
The effect of alpha-amino-3-hydroxy-5-methylisoxazolepropionate (AMPA) on Ca(2+)-sensitive, tetrodotoxin (TTX)-insensitive K(+)-stimulated [3H]-L-glutamate release from rat hippocampal synaptosomes was determined. AMPA in the presence, but not in the absence of cyclothiazide, a drug which blocks AMPA receptor desensitization, elicited a dose-dependent increase in K(+)-stimulated [3H]-L-glutamate release but had no effect on basal release. The AMPA/cyclothiazide stimulation was blocked by CNQX and by GYKI 52466, an antagonist at the cyclothiazide site. These results indicate that AMPA receptors are present on presynaptic terminals and suggest that they may play a role in the regulation of neurotransmitter release.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes J. M., Henley J. M. Molecular characteristics of excitatory amino acid receptors. Prog Neurobiol. 1992 Aug;39(2):113–133. doi: 10.1016/0301-0082(92)90007-2. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Excitotoxic cell death. J Neurobiol. 1992 Nov;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Desai M. A., Burnett J. P., Schoepp D. D. Cyclothiazide selectively potentiates AMPA- and kainate-induced [3H]norepinephrine release from rat hippocampal slices. J Neurochem. 1994 Jul;63(1):231–237. doi: 10.1046/j.1471-4159.1994.63010231.x. [DOI] [PubMed] [Google Scholar]
- Donevan S. D., Rogawski M. A. GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron. 1993 Jan;10(1):51–59. doi: 10.1016/0896-6273(93)90241-i. [DOI] [PubMed] [Google Scholar]
- Marchi M., Bocchieri P., Garbarino L., Raiteri M. Muscarinic inhibition of endogenous glutamate release from rat hippocampus synaptosomes. Neurosci Lett. 1989 Jan 16;96(2):229–234. doi: 10.1016/0304-3940(89)90063-3. [DOI] [PubMed] [Google Scholar]
- McMahon H. T., Barrie A. P., Lowe M., Nicholls D. G. Glutamate release from guinea-pig synaptosomes: stimulation by reuptake-induced depolarization. J Neurochem. 1989 Jul;53(1):71–79. doi: 10.1111/j.1471-4159.1989.tb07296.x. [DOI] [PubMed] [Google Scholar]
- Wenthold R. J., Yokotani N., Doi K., Wada K. Immunochemical characterization of the non-NMDA glutamate receptor using subunit-specific antibodies. Evidence for a hetero-oligomeric structure in rat brain. J Biol Chem. 1992 Jan 5;267(1):501–507. [PubMed] [Google Scholar]
- Zorumski C. F., Yamada K. A., Price M. T., Olney J. W. A benzodiazepine recognition site associated with the non-NMDA glutamate receptor. Neuron. 1993 Jan;10(1):61–67. doi: 10.1016/0896-6273(93)90242-j. [DOI] [PubMed] [Google Scholar]


