Abstract
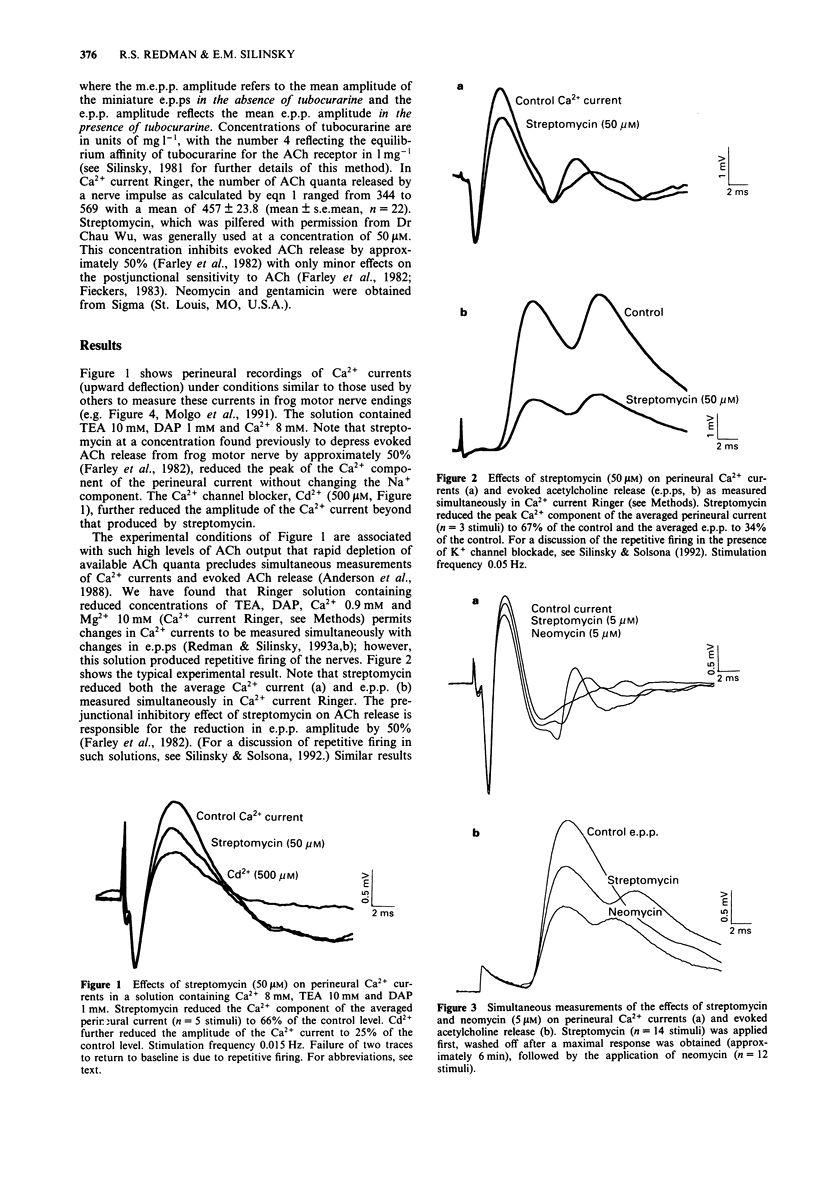
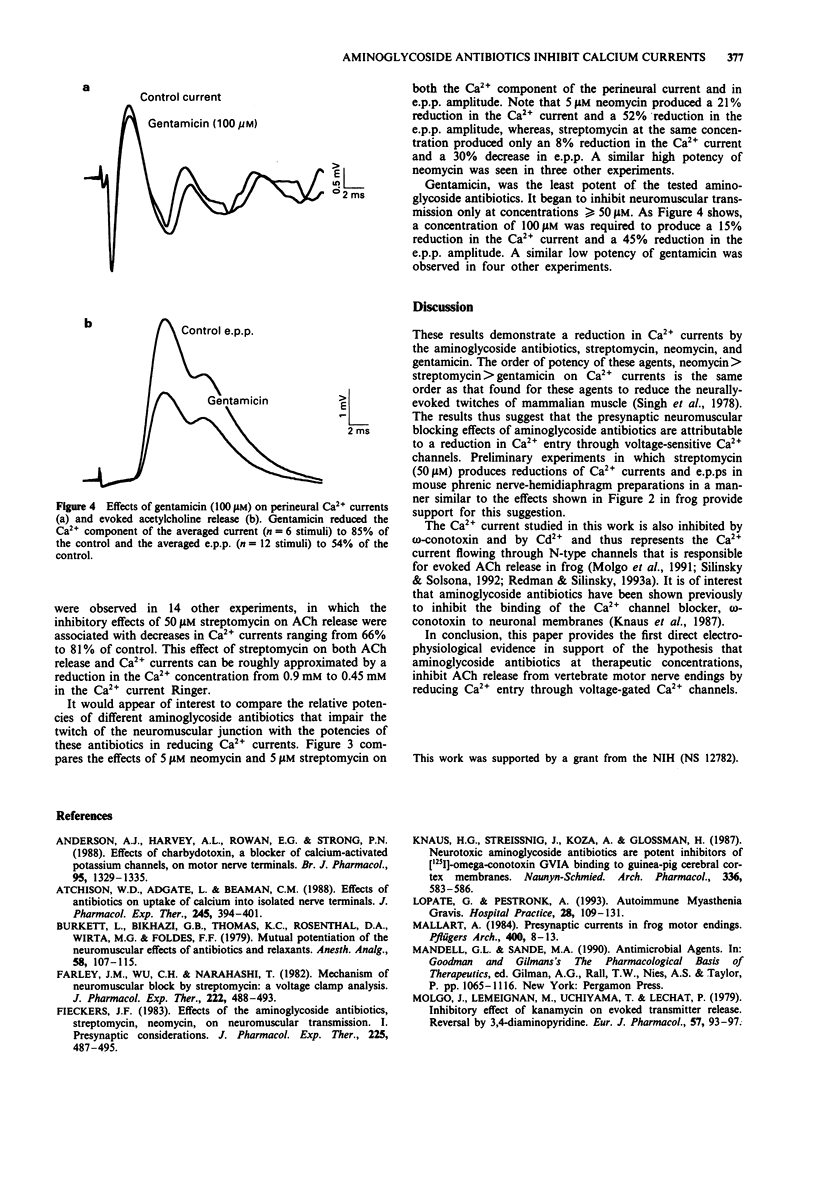
1. The effects of the aminoglycoside antibiotics, streptomycin, neomycin and gentamicin were examined on perineural currents and evoked acetylcholine (ACh) release at frog motor nerve endings. 2. In the standard solutions used previously to measure Ca2+ currents, streptomycin reduced the peak amplitude of the Ca2+ component of the perineural current. 3. In a solution in which changes in both Ca2+ currents and evoked ACh release can be recorded simultaneously, both Ca2+ currents and evoked ACh release were reduced by aminoglycosides in the potency order neomycin > streptomycin > gentamicin. This potency sequence is similar to that reported previously for these agents as inhibitors of neurally-evoked contractions of mammalian skeletal muscle. 4. These data suggest that the presynaptic inhibitory effects of aminoglycoside antibiotics at the neuromuscular junction occur as a consequence of a reduction in Ca2+ currents in the motor nerve terminal.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Harvey A. L., Rowan E. G., Strong P. N. Effects of charybdotoxin, a blocker of Ca2+-activated K+ channels, on motor nerve terminals. Br J Pharmacol. 1988 Dec;95(4):1329–1335. doi: 10.1111/j.1476-5381.1988.tb11772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison W. D., Adgate L., Beaman C. M. Effects of antibiotics on uptake of calcium into isolated nerve terminals. J Pharmacol Exp Ther. 1988 May;245(2):394–401. [PubMed] [Google Scholar]
- Burkett L., Bikhazi G. B., Thomas K. C., Jr, Rosenthal D. A., Wirta M. G., Foldes F. F. Mutual potentiation of the neuromuscular effects of antibiotics and relaxants. Anesth Analg. 1979 Mar-Apr;58(2):107–115. [PubMed] [Google Scholar]
- Farley J. M., Wu C. H., Narahashi T. Mechanism of neuromuscular block by streptomycin: a voltage clamp analysis. J Pharmacol Exp Ther. 1982 Aug;222(2):488–493. [PubMed] [Google Scholar]
- Fiekers J. F. Effects of the aminoglycoside antibiotics, streptomycin and neomycin, on neuromuscular transmission. I. Presynaptic considerations. J Pharmacol Exp Ther. 1983 Jun;225(3):487–495. [PubMed] [Google Scholar]
- Knaus H. G., Striessnig J., Koza A., Glossmann H. Neurotoxic aminoglycoside antibiotics are potent inhibitors of [125I]-Omega-Conotoxin GVIA binding to guinea-pig cerebral cortex membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Nov;336(5):583–586. doi: 10.1007/BF00169318. [DOI] [PubMed] [Google Scholar]
- Lopate G., Pestronk A. Autoimmune myasthenia gravis. Hosp Pract (Off Ed) 1993 Jan 15;28(1):109-12, 115-7, 121-2, passim. doi: 10.1080/21548331.1993.11442741. [DOI] [PubMed] [Google Scholar]
- Mallart A. Presynaptic currents in frog motor endings. Pflugers Arch. 1984 Jan;400(1):8–13. doi: 10.1007/BF00670529. [DOI] [PubMed] [Google Scholar]
- Molgó J., Lemeignan M., Uchiyama T., Lechat P. Inhibitory effect of kanamycin on evoked transmitter release. Reversal by 3,4-diaminopyridine. Eur J Pharmacol. 1979 Jul 15;57(1):93–97. doi: 10.1016/0014-2999(79)90107-9. [DOI] [PubMed] [Google Scholar]
- Molgó J., del Pozo E., Baños J. E., Angaut-Petit D. Changes of quantal transmitter release caused by gadolinium ions at the frog neuromuscular junction. Br J Pharmacol. 1991 Sep;104(1):133–138. doi: 10.1111/j.1476-5381.1991.tb12397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittinger C., Adamson R. Antibiotic blockade of neuromuscular function. Annu Rev Pharmacol. 1972;12:169–184. doi: 10.1146/annurev.pa.12.040172.001125. [DOI] [PubMed] [Google Scholar]
- Redman R. S., Silinsky E. M. A selective adenosine antagonist (8-cyclopentyl-1,3-dipropylxanthine) eliminates both neuromuscular depression and the action of exogenous adenosine by an effect on A1 receptors. Mol Pharmacol. 1993 Oct;44(4):835–840. [PubMed] [Google Scholar]
- Redman R. S., Silinsky E. M. ATP released together with acetylcholine as the mediator of neuromuscular depression at frog motor nerve endings. J Physiol. 1994 May 15;477(Pt 1):117–127. doi: 10.1113/jphysiol.1994.sp020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the calcium receptor that mediates depolarization-secretion coupling at cholinergic motor nerve terminals. Br J Pharmacol. 1981 Jun;73(2):413–429. doi: 10.1111/j.1476-5381.1981.tb10438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Solsona C. S. Calcium currents at motor nerve endings: absence of effects of adenosine receptor agonists in the frog. J Physiol. 1992 Nov;457:315–328. doi: 10.1113/jphysiol.1992.sp019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y. N., Harvey A. L., Marshall I. G. Antibiotic-induced paralysis of the mouse phrenic nerve-hemidiaphragm preparation, and reversibility by calcium and by neostigmine. Anesthesiology. 1978 Jun;48(6):418–424. doi: 10.1097/00000542-197806000-00008. [DOI] [PubMed] [Google Scholar]


