Abstract
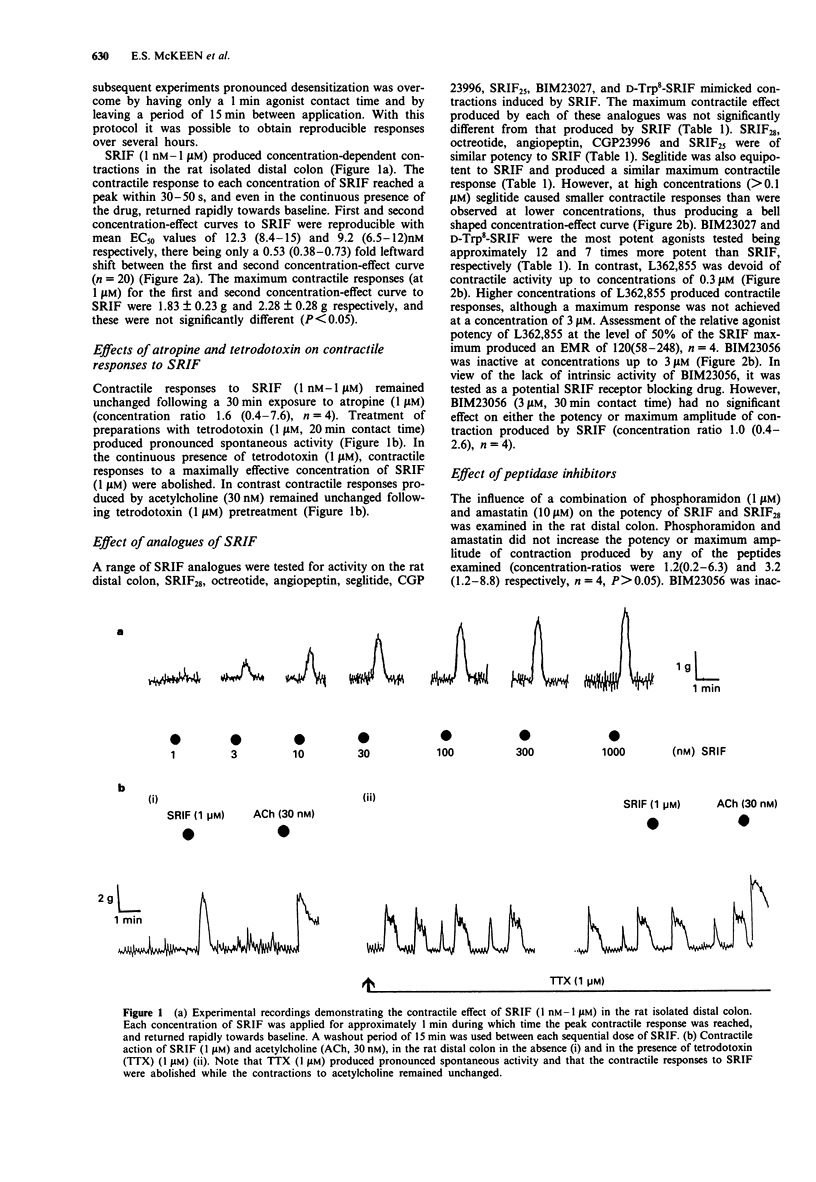
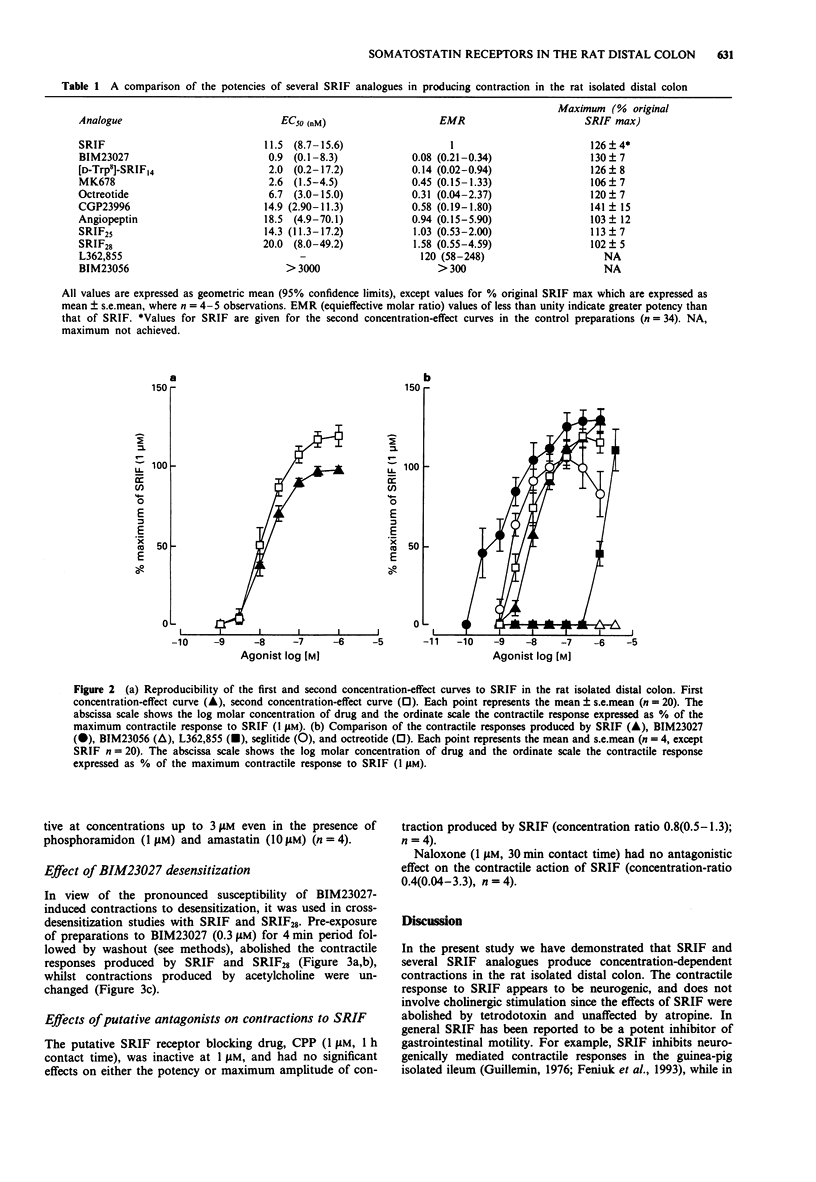
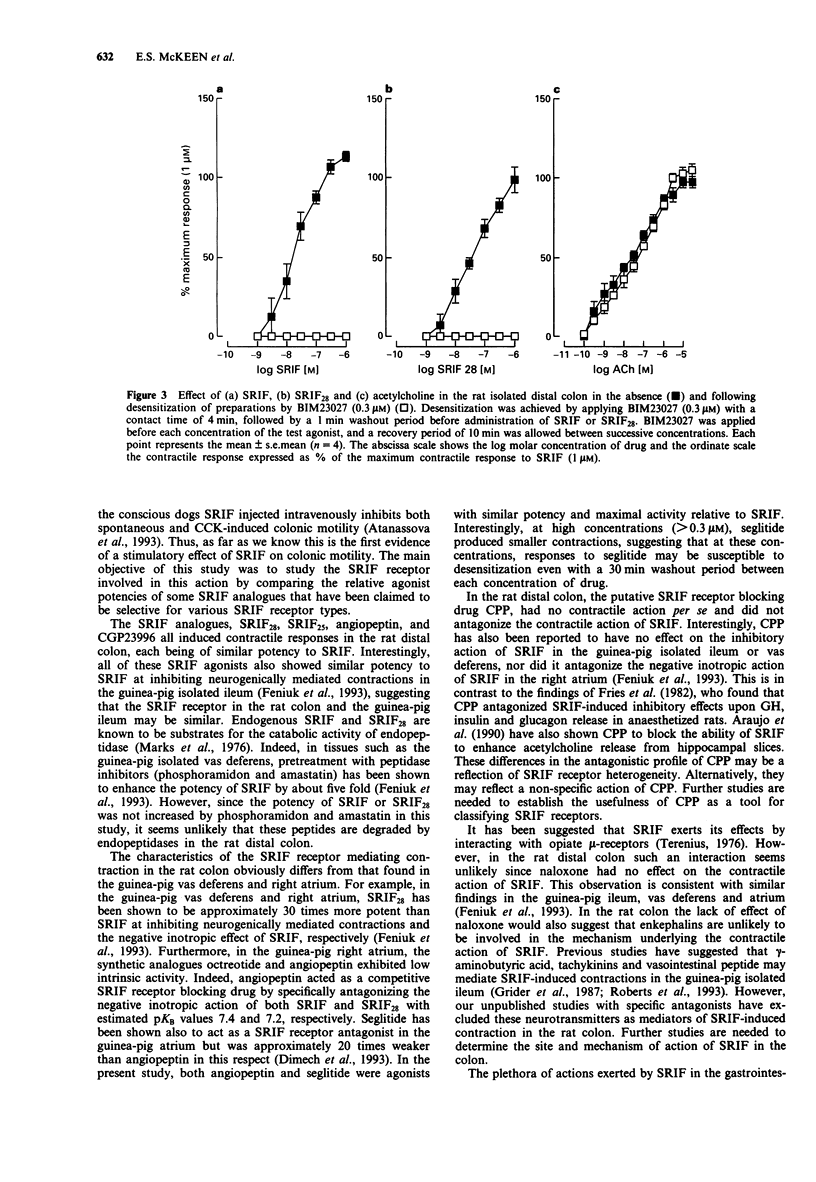
1. The motor effects of somatostatin-14 (SRIF), and several SRIF peptide analogues were investigated on the rat isolated distal colon. The objective of these studies was to characterize the receptor mediating the contractile action of SRIF by comparing the relative agonist potencies of a range of SRIF analogues. 2. SRIF (1 nM-1 microM) produced concentration-dependent contractions with an EC50 value of approximately 10 nM. Contractile responses induced by SRIF were insensitive to atropine (1 microM) or naloxone (1 microM) but abolished by tetrodotoxin (1 microM). Somatostatin-28 (SRIF28), also induced concentration-dependent contractions and was equipotent with SRIF. Phosphoramidon (1 microM) and amastatin (10 microM) did not increase the potency of either SRIF or SRIF28. 3. The SRIF peptide analogues, octreotide, SRIF25, seglitide, angiopeptin and CGP23996 (1 nM-1 microM) produced contractile responses in the rat distal colon, each having similar potency and maximal activity relative to SRIF. The SSTR2 receptor-selective hexapeptide, BIM23027 (0.1 nM-1 microM), and the SRIF stereoisomer, D-Trp8-SRIF (0.1 nM-1 microM), were the most potent agonists examined being approximately 12 and 7 times more potent than SRIF, respectively. In contrast, the SSTR5 receptor-selective analogue, L362,855, was approximately 120 times weaker than SRIF, whilst the SSTR3 receptor-selective analogue, BIM23056, was inactive at concentrations up to 3 microM. 4. The putative SRIF receptor antagonist, (cyclo(7-aminoheptanoyl Phe-D-Trp-Lys-Thr[Bzl]))(CPP) (1 microM), had no agonist activity and had no effect on contractions induced by SRIF. 5. The contractile actions of BIM23027 and seglitide were subject to pronounced desensitization.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo D. M., Lapchak P. A., Collier B., Quirion R. Evidence that somatostatin enhances endogenous acetylcholine release in the rat hippocampus. J Neurochem. 1990 Nov;55(5):1546–1555. doi: 10.1111/j.1471-4159.1990.tb04937.x. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Reisine T. Molecular biology of somatostatin receptors. Trends Neurosci. 1993 Jan;16(1):34–38. doi: 10.1016/0166-2236(93)90050-v. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Ling N., Esch F., Böhlen P., Benoit R., Guillemin R. High biological activity of the synthetic replicates of somatostatin-28 and somatostatin-25. Regul Pept. 1981 Jan;1(4):255–264. doi: 10.1016/0167-0115(81)90048-3. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Bruno J. F., Xu Y., Song J., Berelowitz M. Tissue distribution of somatostatin receptor subtype messenger ribonucleic acid in the rat. Endocrinology. 1993 Dec;133(6):2561–2567. doi: 10.1210/endo.133.6.8243278. [DOI] [PubMed] [Google Scholar]
- Chao A. C., Zifferblatt J. B., Wagner J. A., Dong Y. J., Gruenert D. C., Gardner P. Stimulation of chloride secretion by P1 purinoceptor agonists in cystic fibrosis phenotype airway epithelial cell line CFPEo-. Br J Pharmacol. 1994 May;112(1):169–175. doi: 10.1111/j.1476-5381.1994.tb13047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corness J. D., Demchyshyn L. L., Seeman P., Van Tol H. H., Srikant C. B., Kent G., Patel Y. C., Niznik H. B. A human somatostatin receptor (SSTR3), located on chromosome 22, displays preferential affinity for somatostatin-14 like peptides. FEBS Lett. 1993 Apr 26;321(2-3):279–284. doi: 10.1016/0014-5793(93)80124-d. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B., Smith I. J., Davies B., Oliver J. An immunohistochemical study of the projections of somatostatin-containing neurons in the guinea-pig intestine. Neuroscience. 1980;5(5):841–852. doi: 10.1016/0306-4522(80)90153-0. [DOI] [PubMed] [Google Scholar]
- Dimech J., Feniuk W., Humphrey P. P. Antagonist effects of seglitide (MK 678) at somatostatin receptors in guinea-pig isolated right atria. Br J Pharmacol. 1993 Aug;109(4):898–899. doi: 10.1111/j.1476-5381.1993.tb13703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E., Ekman R., Håkanson R., Sundler F. Projections of peptide-containing neurons in rat colon. Neuroscience. 1988 Nov;27(2):655–674. doi: 10.1016/0306-4522(88)90296-5. [DOI] [PubMed] [Google Scholar]
- Feniuk W., Dimech J., Humphrey P. P. Characterization of somatostatin receptors in guinea-pig isolated ileum, vas deferens and right atrium. Br J Pharmacol. 1993 Nov;110(3):1156–1164. doi: 10.1111/j.1476-5381.1993.tb13935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries J. L., Murphy W. A., Sueiras-Diaz J., Coy D. H. Somatostatin antagonist analog increases GH, insulin, and glucagon release in the rat. Peptides. 1982 Sep-Oct;3(5):811–814. doi: 10.1016/0196-9781(82)90020-1. [DOI] [PubMed] [Google Scholar]
- Grider J. R., Arimura A., Makhlouf G. M. Role of somatostatin neurons in intestinal peristalsis: facilitatory interneurons in descending pathways. Am J Physiol. 1987 Oct;253(4 Pt 1):G434–G438. doi: 10.1152/ajpgi.1987.253.4.G434. [DOI] [PubMed] [Google Scholar]
- Gu Z. F., Pradhan T., Coy D. H., Mantey S., Bunnett N. W., Jensen R. T., Maton P. N. Actions of somatostatins on gastric smooth muscle cells. Am J Physiol. 1992 Mar;262(3 Pt 1):G432–G438. doi: 10.1152/ajpgi.1992.262.3.G432. [DOI] [PubMed] [Google Scholar]
- Gyr K. E., Meier R. Pharmacodynamic effects of Sandostatin in the gastrointestinal tract. Digestion. 1993;54 (Suppl 1):14–19. doi: 10.1159/000201070. [DOI] [PubMed] [Google Scholar]
- Hirst B. H., Conlon J. M., Coy D. H., Holland J., Shaw B. Comparison of the gastric exocrine inhibitory activities and plasma kinetics of somatostatin-28 and somatostatin-14 in cats. Regul Pept. 1982 Sep;4(4):227–237. doi: 10.1016/0167-0115(82)90115-x. [DOI] [PubMed] [Google Scholar]
- Keast J. R., Furness J. B., Costa M. Somatostatin in human enteric nerves. Distribution and characterization. Cell Tissue Res. 1984;237(2):299–308. doi: 10.1007/BF00217149. [DOI] [PubMed] [Google Scholar]
- Kluxen F. W., Bruns C., Lübbert H. Expression cloning of a rat brain somatostatin receptor cDNA. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4618–4622. doi: 10.1073/pnas.89.10.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. J., Forte M., North R. A., Ross C. A., Snyder S. H. Cloning and expression of a rat somatostatin receptor enriched in brain. J Biol Chem. 1992 Oct 25;267(30):21307–21312. [PubMed] [Google Scholar]
- Marks N., Stern F., Benuck M. Correlation between biological potency and biodegradation of a somatostatin analogue. Nature. 1976 Jun 10;261(5560):511–512. doi: 10.1038/261511a0. [DOI] [PubMed] [Google Scholar]
- Melvin L. S., Milne G. M., Johnson M. R., Subramaniam B., Wilken G. H., Howlett A. C. Structure-activity relationships for cannabinoid receptor-binding and analgesic activity: studies of bicyclic cannabinoid analogs. Mol Pharmacol. 1993 Nov;44(5):1008–1015. [PubMed] [Google Scholar]
- Meyerhof W., Wulfsen I., Schönrock C., Fehr S., Richter D. Molecular cloning of a somatostatin-28 receptor and comparison of its expression pattern with that of a somatostatin-14 receptor in rat brain. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10267–10271. doi: 10.1073/pnas.89.21.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W., Wulfsen I., Schönrock C., Fehr S., Richter D. Molecular cloning of a somatostatin-28 receptor and comparison of its expression pattern with that of a somatostatin-14 receptor in rat brain. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10267–10271. doi: 10.1073/pnas.89.21.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers C. A., Murphy W. A., Redding T. W., Coy D. H., Schally A. V. Synthesis and biological actions of prosomatostatin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6171–6174. doi: 10.1073/pnas.77.10.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll A. M., Lolait S. J., König M., Mahan L. C. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol Pharmacol. 1992 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Ormsbee H. S., 3rd, Koehler S. L., Jr, Telford G. L. Somatostatin inhibits motilin-induced interdigestive contractile activity in the dog. Am J Dig Dis. 1978 Sep;23(9):781–788. doi: 10.1007/BF01079786. [DOI] [PubMed] [Google Scholar]
- Peeters T. L., Janssens J., Vantrappen G. R. Somatostatin and the interdigestive migrating motor complex in man. Regul Pept. 1983 Feb;5(3):209–217. doi: 10.1016/0167-0115(83)90252-5. [DOI] [PubMed] [Google Scholar]
- Pradayrol L., Jörnvall H., Mutt V., Ribet A. N-terminally extended somatostatin: the primary structure of somatostatin-28. FEBS Lett. 1980 Jan 1;109(1):55–58. doi: 10.1016/0014-5793(80)81310-x. [DOI] [PubMed] [Google Scholar]
- Raynor K., Murphy W. A., Coy D. H., Taylor J. E., Moreau J. P., Yasuda K., Bell G. I., Reisine T. Cloned somatostatin receptors: identification of subtype-selective peptides and demonstration of high affinity binding of linear peptides. Mol Pharmacol. 1993 Jun;43(6):838–844. [PubMed] [Google Scholar]
- Raynor K., O'Carroll A. M., Kong H., Yasuda K., Mahan L. C., Bell G. I., Reisine T. Characterization of cloned somatostatin receptors SSTR4 and SSTR5. Mol Pharmacol. 1993 Aug;44(2):385–392. [PubMed] [Google Scholar]
- Raynor K., Reisine T. Differential coupling of somatostatin1 receptors to adenylyl cyclase in the rat striatum vs. the pituitary and other regions of the rat brain. J Pharmacol Exp Ther. 1992 Feb;260(2):841–848. [PubMed] [Google Scholar]
- Raynor K., Reisine T. Subtypes of somatostatin receptors are expressed in the anterior pituitary cell line GH3. J Pharmacol Exp Ther. 1993 Jan;264(1):110–116. [PubMed] [Google Scholar]
- Reisine T. Multiple mechanisms of somatostatin inhibition of adrenocorticotropin release from mouse anterior pituitary tumor cells. Endocrinology. 1985 Jun;116(6):2259–2266. doi: 10.1210/endo-116-6-2259. [DOI] [PubMed] [Google Scholar]
- Rens-Domiano S., Law S. F., Yamada Y., Seino S., Bell G. I., Reisine T. Pharmacological properties of two cloned somatostatin receptors. Mol Pharmacol. 1992 Jul;42(1):28–34. [PubMed] [Google Scholar]
- Roberts D. J., Hasler W. L., Owyang C. GABA mediation of the dual effects of somatostatin on guinea pig ileal myenteric cholinergic transmission. Am J Physiol. 1993 May;264(5 Pt 1):G953–G960. doi: 10.1152/ajpgi.1993.264.5.G953. [DOI] [PubMed] [Google Scholar]
- Shen K. Z., Surprenant A. Somatostatin-mediated inhibitory postsynaptic potential in sympathetically denervated guinea-pig submucosal neurones. J Physiol. 1993 Oct;470:619–635. doi: 10.1113/jphysiol.1993.sp019878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius L. Somatostatin and ACTH are peptides with partial antagonist-like selectivity for opiate receptors. Eur J Pharmacol. 1976 Jul;38(1):211–213. doi: 10.1016/0014-2999(76)90221-1. [DOI] [PubMed] [Google Scholar]
- Thor P., Król R., Konturek S. J., Coy D. H., Schally A. V. Effect of somatostatin on myoelectrical activity of small bowel. Am J Physiol. 1978 Sep;235(3):E249–E254. doi: 10.1152/ajpendo.1978.235.3.E249. [DOI] [PubMed] [Google Scholar]
- Tran V. T., Beal M. F., Martin J. B. Two types of somatostatin receptors differentiated by cyclic somatostatin analogs. Science. 1985 Apr 26;228(4698):492–495. doi: 10.1126/science.2858917. [DOI] [PubMed] [Google Scholar]
- Vanetti M., Kouba M., Wang X., Vogt G., Höllt V. Cloning and expression of a novel mouse somatostatin receptor (SSTR2B). FEBS Lett. 1992 Oct 26;311(3):290–294. doi: 10.1016/0014-5793(92)81122-3. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Post S. R., Wang K., Tager H. S., Bell G. I., Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Reisine T., Law S. F., Ihara Y., Kubota A., Kagimoto S., Seino M., Seino Y., Bell G. I., Seino S. Somatostatin receptors, an expanding gene family: cloning and functional characterization of human SSTR3, a protein coupled to adenylyl cyclase. Mol Endocrinol. 1992 Dec;6(12):2136–2142. doi: 10.1210/mend.6.12.1337145. [DOI] [PubMed] [Google Scholar]
- Yasuda K., Rens-Domiano S., Breder C. D., Law S. F., Saper C. B., Reisine T., Bell G. I. Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J Biol Chem. 1992 Oct 5;267(28):20422–20428. [PubMed] [Google Scholar]


