Abstract
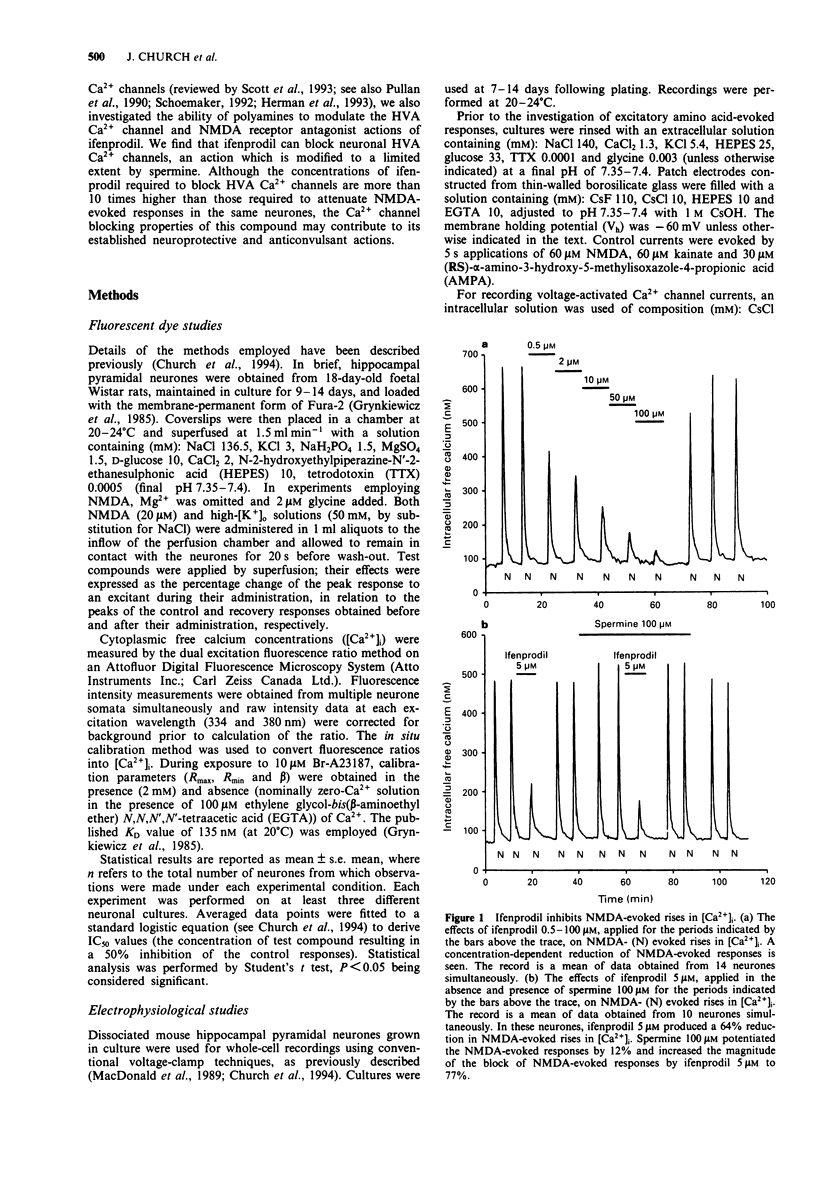
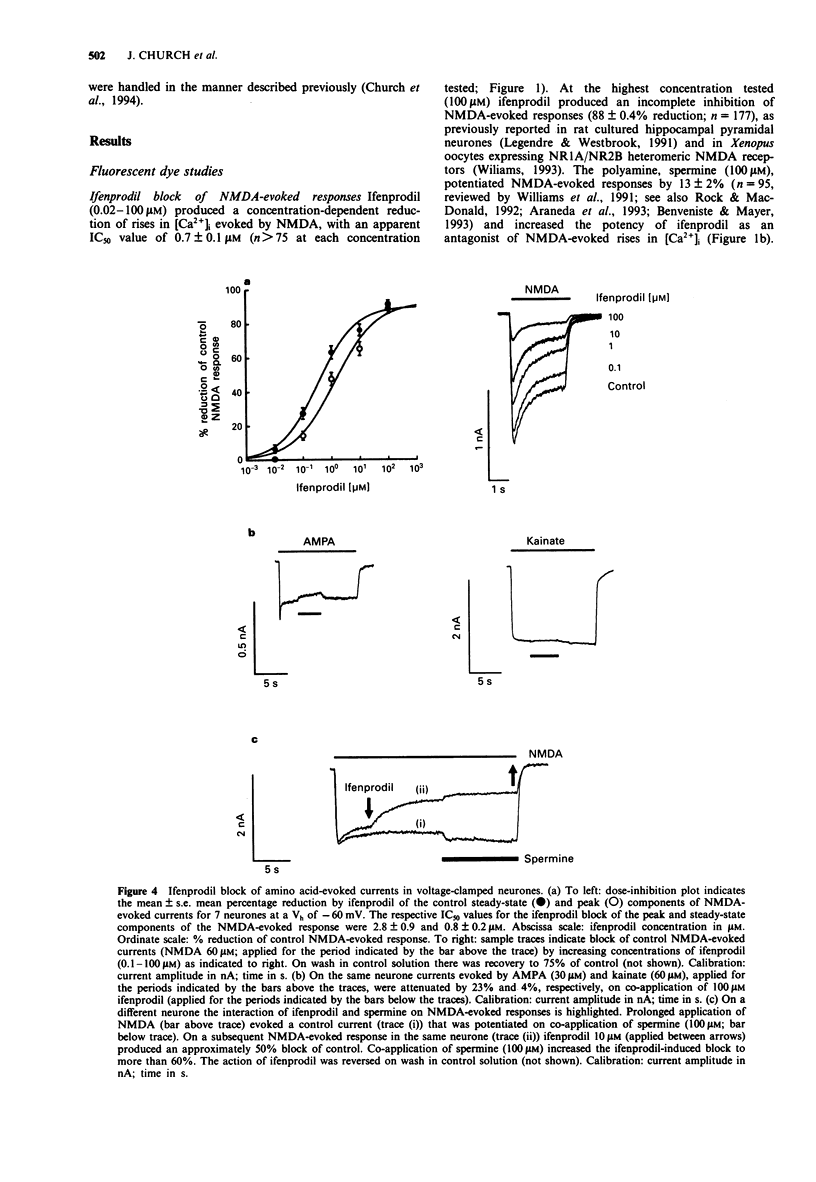
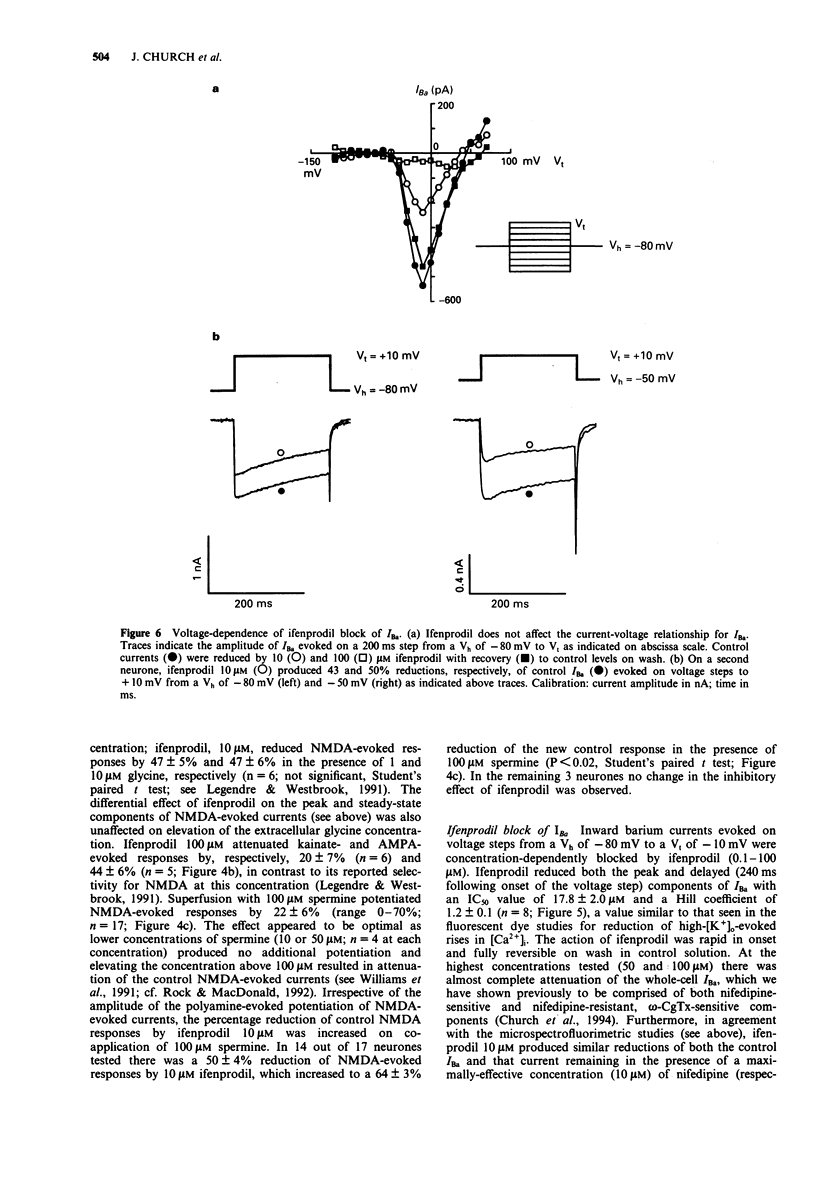
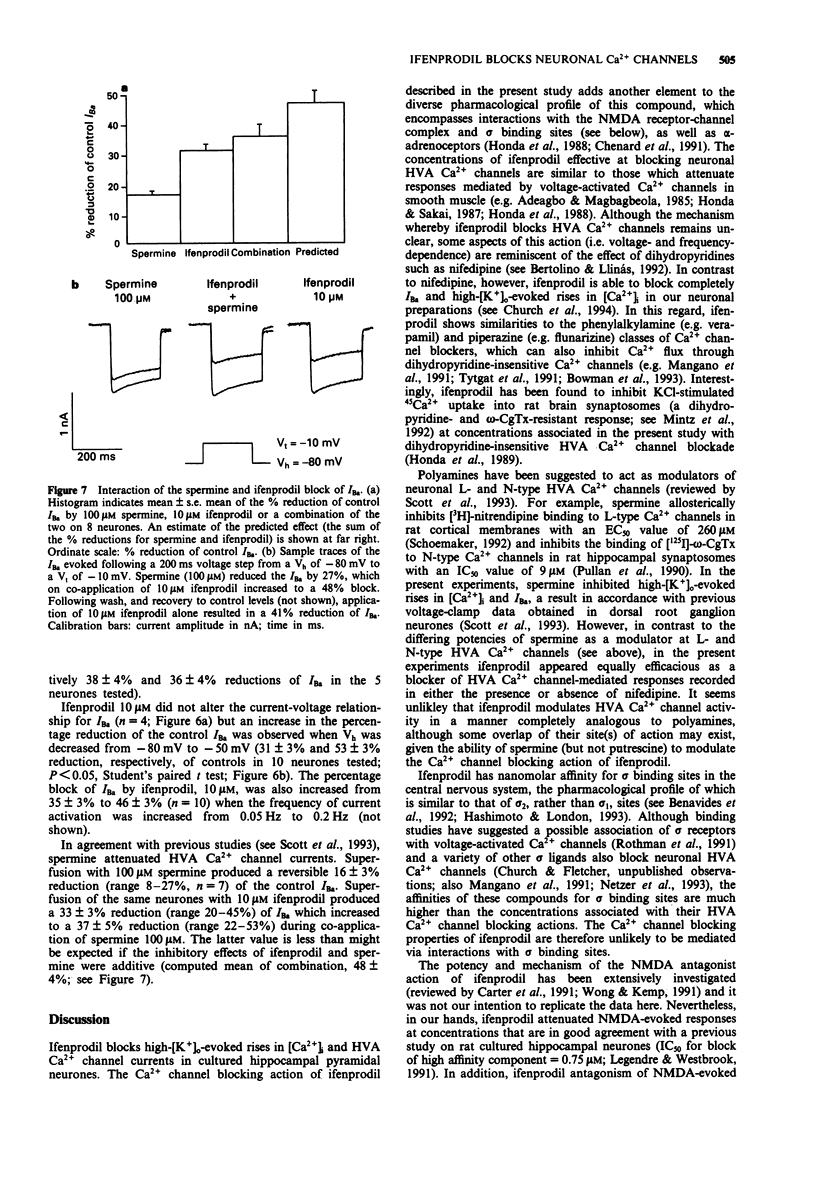
1. The block by ifenprodil of voltage-activated Ca2+ channels was investigated in intracellular free calcium concentration ([Ca2+]i) evoked by 50 mM K+ (high-[K+]o) in Fura-2-loaded rat hippocampal pyramidal neurones in culture and on currents carried by Ba2+ ions (IBa) through Ca2+ channels in mouse cultured hippocampal neurones under whole-cell voltage-clamp. The effects of ifenprodil on voltage-activated Ca2+ channels were compared with its antagonist actions on N-methyl-D-aspartate- (NMDA) evoked responses in the same neuronal preparations. 2. Rises in [Ca2+]i evoked by transient exposure to high-[K+]o in our preparation of rat cultured hippocampal pyramidal neurones are mediated predominantly by Ca2+ flux through nifedipine-sensitive Ca2+ channels, with smaller contributions from nifedipine-resistant, omega-conotoxin GVIA-sensitive Ca2+ channels and Ca2+ channels sensitive to crude funnel-web spider venom (Church et al., 1994). Ifenprodil (0.1-200 microM) reversibly attenuated high-[K+]o-evoked rises in [Ca2+]i with an IC50 value of 17 +/- 3 microM, compared with an IC50 value of 0.7 +/- 0.1 microM for the reduction of rises in [Ca2+]i evoked by 20 microM NMDA. Tested in the presence of nifedipine 10 microM, ifenprodil (1-50 microM) produced a concentration-dependent reduction of the dihydropyridine-resistant high-[K+]o-evoked rise in [Ca2+]i with an IC50 value of 13 +/- 4 microM. The results suggest that ifenprodil blocks Ca2+ flux through multiple subtypes of high voltage-activated Ca2+ channels. 3. Application of the polyamine, spermine (0.25-5 mM), produced a concentration-dependent reduction of rises in [Ca2+]i evoked by high-[K+]o.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeagbo A. S., Magbagbeola A. O. Pharmacological actions of ifenprodil in the rat isolated anococcygeus muscle. J Pharm Pharmacol. 1985 Nov;37(11):833–835. doi: 10.1111/j.2042-7158.1985.tb04982.x. [DOI] [PubMed] [Google Scholar]
- Annels S. J., Ellis Y., Davies J. A. Non-opioid antitussives inhibit endogenous glutamate release from rabbit hippocampal slices. Brain Res. 1991 Nov 15;564(2):341–343. doi: 10.1016/0006-8993(91)91474-f. [DOI] [PubMed] [Google Scholar]
- Araneda R. C., Zukin R. S., Bennett M. V. Effects of polyamines on NMDA-induced currents in rat hippocampal neurons: a whole-cell and single-channel study. Neurosci Lett. 1993 Apr 2;152(1-2):107–112. doi: 10.1016/0304-3940(93)90495-7. [DOI] [PubMed] [Google Scholar]
- Bakker M. H., McKernan R. M., Wong E. H., Foster A. C. [3H]MK-801 binding to N-methyl-D-aspartate receptors solubilized from rat brain: effects of glycine site ligands, polyamines, ifenprodil, and desipramine. J Neurochem. 1991 Jul;57(1):39–45. doi: 10.1111/j.1471-4159.1991.tb02096.x. [DOI] [PubMed] [Google Scholar]
- Benavides J., Peny B., Allen J., Scatton B. Pharmacological characterization of in vivo [3H]lfenprodil binding sites in the mouse brain. J Pharmacol Exp Ther. 1992 Feb;260(2):896–901. [PubMed] [Google Scholar]
- Benveniste M., Mayer M. L. Multiple effects of spermine on N-methyl-D-aspartic acid receptor responses of rat cultured hippocampal neurones. J Physiol. 1993 May;464:131–163. doi: 10.1113/jphysiol.1993.sp019627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino M., Llinás R. R. The central role of voltage-activated and receptor-operated calcium channels in neuronal cells. Annu Rev Pharmacol Toxicol. 1992;32:399–421. doi: 10.1146/annurev.pa.32.040192.002151. [DOI] [PubMed] [Google Scholar]
- Bowman D., Alexander S., Lodge D. Pharmacological characterisation of the calcium channels coupled to the plateau phase of KCl-induced intracellular free Ca2+ elevation in chicken and rat synaptosomes. Neuropharmacology. 1993 Nov;32(11):1195–1202. doi: 10.1016/0028-3908(93)90013-s. [DOI] [PubMed] [Google Scholar]
- Chenard B. L., Shalaby I. A., Koe B. K., Ronau R. T., Butler T. W., Prochniak M. A., Schmidt A. W., Fox C. B. Separation of alpha 1 adrenergic and N-methyl-D-aspartate antagonist activity in a series of ifenprodil compounds. J Med Chem. 1991 Oct;34(10):3085–3090. doi: 10.1021/jm00114a018. [DOI] [PubMed] [Google Scholar]
- Church J., Fletcher E. J., Abdel-Hamid K., MacDonald J. F. Loperamide blocks high-voltage-activated calcium channels and N-methyl-D-aspartate-evoked responses in rat and mouse cultured hippocampal pyramidal neurons. Mol Pharmacol. 1994 Apr;45(4):747–757. [PubMed] [Google Scholar]
- Cousin M. A., Nicholls D. G., Pocock J. M. Flunarizine inhibits both calcium-dependent and -independent release of glutamate from synaptosomes and cultured neurones. Brain Res. 1993 Mar 26;606(2):227–236. doi: 10.1016/0006-8993(93)90989-z. [DOI] [PubMed] [Google Scholar]
- Czuczwar S. J., Gasior M., Janusz W., Kleinrok Z. Influence of flunarizine, nicardipine and nimodipine on the anticonvulsant activity of different antiepileptic drugs in mice. Neuropharmacology. 1992 Nov;31(11):1179–1183. doi: 10.1016/0028-3908(92)90015-h. [DOI] [PubMed] [Google Scholar]
- De Sarro G. B., De Sarro A. Anticonvulsant properties of non-competitive antagonists of the N-methyl-D-aspartate receptor in genetically epilepsy-prone rats: comparison with CPPene. Neuropharmacology. 1993 Jan;32(1):51–58. doi: 10.1016/0028-3908(93)90129-q. [DOI] [PubMed] [Google Scholar]
- Deshpande J. K., Wieloch T. Flunarizine, a calcium entry blocker, ameliorates ischemic brain damage in the rat. Anesthesiology. 1986 Feb;64(2):215–224. doi: 10.1097/00000542-198602000-00015. [DOI] [PubMed] [Google Scholar]
- Durand G. M., Gregor P., Zheng X., Bennett M. V., Uhl G. R., Zukin R. S. Cloning of an apparent splice variant of the rat N-methyl-D-aspartate receptor NMDAR1 with altered sensitivity to polyamines and activators of protein kinase C. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9359–9363. doi: 10.1073/pnas.89.19.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Mullen J. M., Rogawski M. A. Phencyclidine block of calcium current in isolated guinea-pig hippocampal neurones. J Physiol. 1992 Oct;456:85–105. doi: 10.1113/jphysiol.1992.sp019328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hashimoto K., London E. D. Further characterization of [3H]ifenprodil binding to sigma receptors in rat brain. Eur J Pharmacol. 1993 May 12;236(1):159–163. doi: 10.1016/0014-2999(93)90241-9. [DOI] [PubMed] [Google Scholar]
- Heinemann U., Hamon B. Calcium and epileptogenesis. Exp Brain Res. 1986;65(1):1–10. doi: 10.1007/BF00243826. [DOI] [PubMed] [Google Scholar]
- Herman M. D., Reuveny E., Narahashi T. The effect of polyamines on voltage-activated calcium channels in mouse neuroblastoma cells. J Physiol. 1993 Mar;462:645–660. doi: 10.1113/jphysiol.1993.sp019574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H., Sakai Y., Iwata T., Ohba S., Kanezuka T., Irino O. Effects of ifenprodil tartrate on alpha-adrenoceptors and Ca2+ movement in isolated canine saphenous veins. Arch Int Pharmacodyn Ther. 1988 Mar-Apr;292:112–121. [PubMed] [Google Scholar]
- Honda H., Sakai Y. The mode of action of ifenprodil tartrate in isolated canine cerebral and femoral arteries. Arch Int Pharmacodyn Ther. 1987 Feb;285(2):211–225. [PubMed] [Google Scholar]
- Honda H., Shibuya T., Salafsky B. Effects of ifenprodil tartrate on calcium flux in arteries and brain synaptosomes. Proc West Pharmacol Soc. 1989;32:155–158. [PubMed] [Google Scholar]
- Legendre P., Westbrook G. L. Ifenprodil blocks N-methyl-D-aspartate receptors by a two-component mechanism. Mol Pharmacol. 1991 Aug;40(2):289–298. [PubMed] [Google Scholar]
- Luebke J. I., Dunlap K., Turner T. J. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993 Nov;11(5):895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- Löscher W., Hönack D. Differences in anticonvulsant potency and adverse effects between dextromethorphan and dextrorphan in amygdala-kindled and non-kindled rats. Eur J Pharmacol. 1993 Jul 20;238(2-3):191–200. doi: 10.1016/0014-2999(93)90847-b. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Bartlett M. C., Mody I., Pahapill P., Reynolds J. N., Salter M. W., Schneiderman J. H., Pennefather P. S. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J Physiol. 1991 Jan;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Mody I., Salter M. W. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989 Jul;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano T. J., Patel J., Salama A. I., Keith R. A. Inhibition of K(+)-evoked [3H]D-aspartate release and neuronal calcium influx by verapamil, diltiazem and dextromethorphan: evidence for non-L/non-N voltage-sensitive calcium channels. Eur J Pharmacol. 1991 Jan 3;192(1):9–17. doi: 10.1016/0014-2999(91)90062-u. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Swiderek K. M., Lee T. D., Bean B. P., Adams M. E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992 Feb 27;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Netzer R., Pflimlin P., Trube G. Dextromethorphan blocks N-methyl-D-aspartate-induced currents and voltage-operated inward currents in cultured cortical neurons. Eur J Pharmacol. 1993 Jul 20;238(2-3):209–216. doi: 10.1016/0014-2999(93)90849-d. [DOI] [PubMed] [Google Scholar]
- Prince D. A., Feeser H. R. Dextromethorphan protects against cerebral infarction in a rat model of hypoxia-ischemia. Neurosci Lett. 1988 Mar 10;85(3):291–296. doi: 10.1016/0304-3940(88)90581-2. [DOI] [PubMed] [Google Scholar]
- Pullan L. M., Keith R. A., LaMonte D., Stumpo R. J., Salama A. I. The polyamine spermine affects omega-conotoxin binding and function at N-type voltage-sensitive calcium channels. J Auton Pharmacol. 1990 Aug;10(4):213–219. doi: 10.1111/j.1474-8673.1990.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Ransom R. W., Stec N. L. Cooperative modulation of [3H]MK-801 binding to the N-methyl-D-aspartate receptor-ion channel complex by L-glutamate, glycine, and polyamines. J Neurochem. 1988 Sep;51(3):830–836. doi: 10.1111/j.1471-4159.1988.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Miller R. J. Ifenprodil is a novel type of N-methyl-D-aspartate receptor antagonist: interaction with polyamines. Mol Pharmacol. 1989 Nov;36(5):758–765. [PubMed] [Google Scholar]
- Rock D. M., Macdonald R. L. The polyamine spermine has multiple actions on N-methyl-D-aspartate receptor single-channel currents in cultured cortical neurons. Mol Pharmacol. 1992 Jan;41(1):83–88. [PubMed] [Google Scholar]
- Rothman R. B., Reid A., Mahboubi A., Kim C. H., De Costa B. R., Jacobson A. E., Rice K. C. Labeling by [3H]1,3-di(2-tolyl)guanidine of two high affinity binding sites in guinea pig brain: evidence for allosteric regulation by calcium channel antagonists and pseudoallosteric modulation by sigma ligands. Mol Pharmacol. 1991 Feb;39(2):222–232. [PubMed] [Google Scholar]
- Schoemaker H., Allen J., Langer S. Z. Binding of [3H]ifenprodil, a novel NMDA antagonist, to a polyamine-sensitive site in the rat cerebral cortex. Eur J Pharmacol. 1990 Feb 6;176(2):249–250. doi: 10.1016/0014-2999(90)90539-i. [DOI] [PubMed] [Google Scholar]
- Schoemaker H. Polyamines allosterically modulate [3H]nitrendipine binding to the voltage-sensitive calcium channel in rat brain. Eur J Pharmacol. 1992 Feb 13;225(2):167–169. doi: 10.1016/0922-4106(92)90097-f. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Sutton K. G., Dolphin A. C. Interactions of polyamines with neuronal ion channels. Trends Neurosci. 1993 Apr;16(4):153–160. doi: 10.1016/0166-2236(93)90124-5. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab. 1989 Apr;9(2):127–140. doi: 10.1038/jcbfm.1989.20. [DOI] [PubMed] [Google Scholar]
- Tytgat J., Pauwels P. J., Vereecke J., Carmeliet E. Flunarizine inhibits a high-threshold inactivating calcium channel (N-type) in isolated hippocampal neurons. Brain Res. 1991 May 17;549(1):112–117. doi: 10.1016/0006-8993(91)90606-v. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993 Oct;44(4):851–859. [PubMed] [Google Scholar]
- Williams K., Romano C., Dichter M. A., Molinoff P. B. Modulation of the NMDA receptor by polyamines. Life Sci. 1991;48(6):469–498. doi: 10.1016/0024-3205(91)90463-l. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A. Sites for antagonism on the N-methyl-D-aspartate receptor channel complex. Annu Rev Pharmacol Toxicol. 1991;31:401–425. doi: 10.1146/annurev.pa.31.040191.002153. [DOI] [PubMed] [Google Scholar]


