Abstract
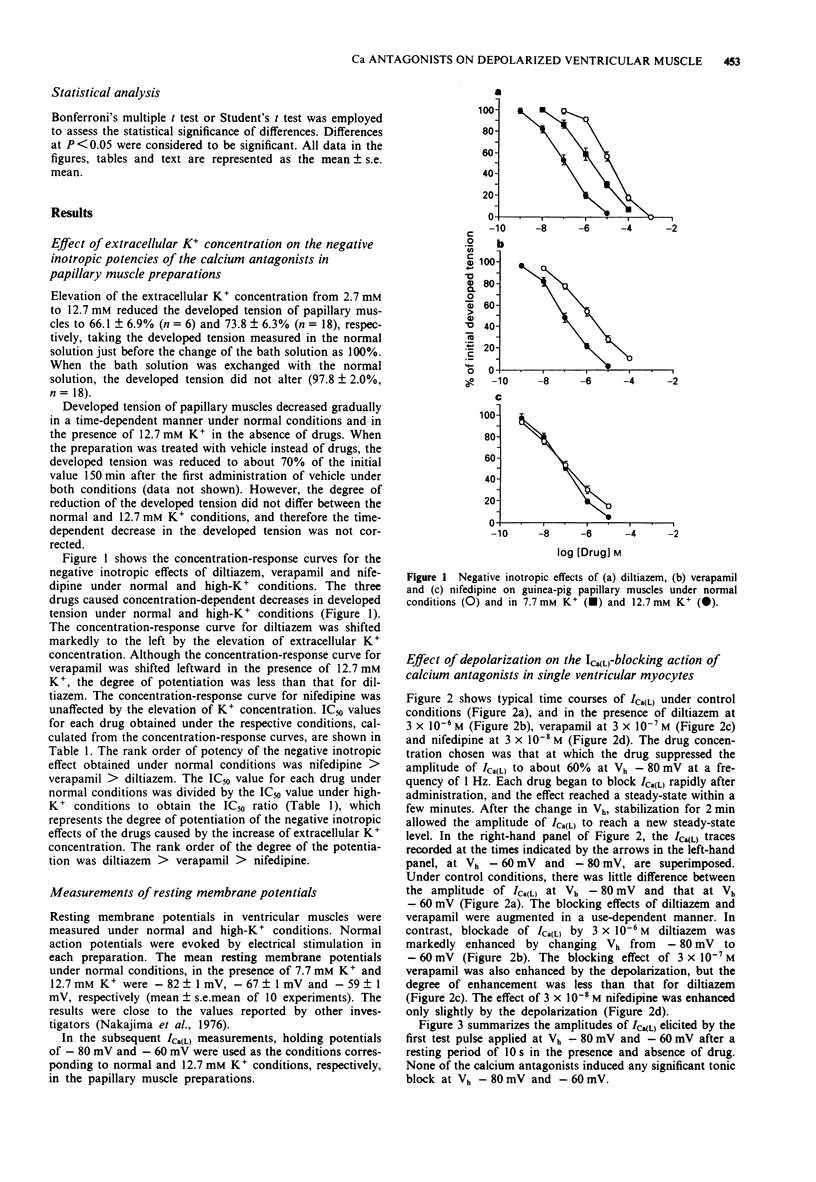
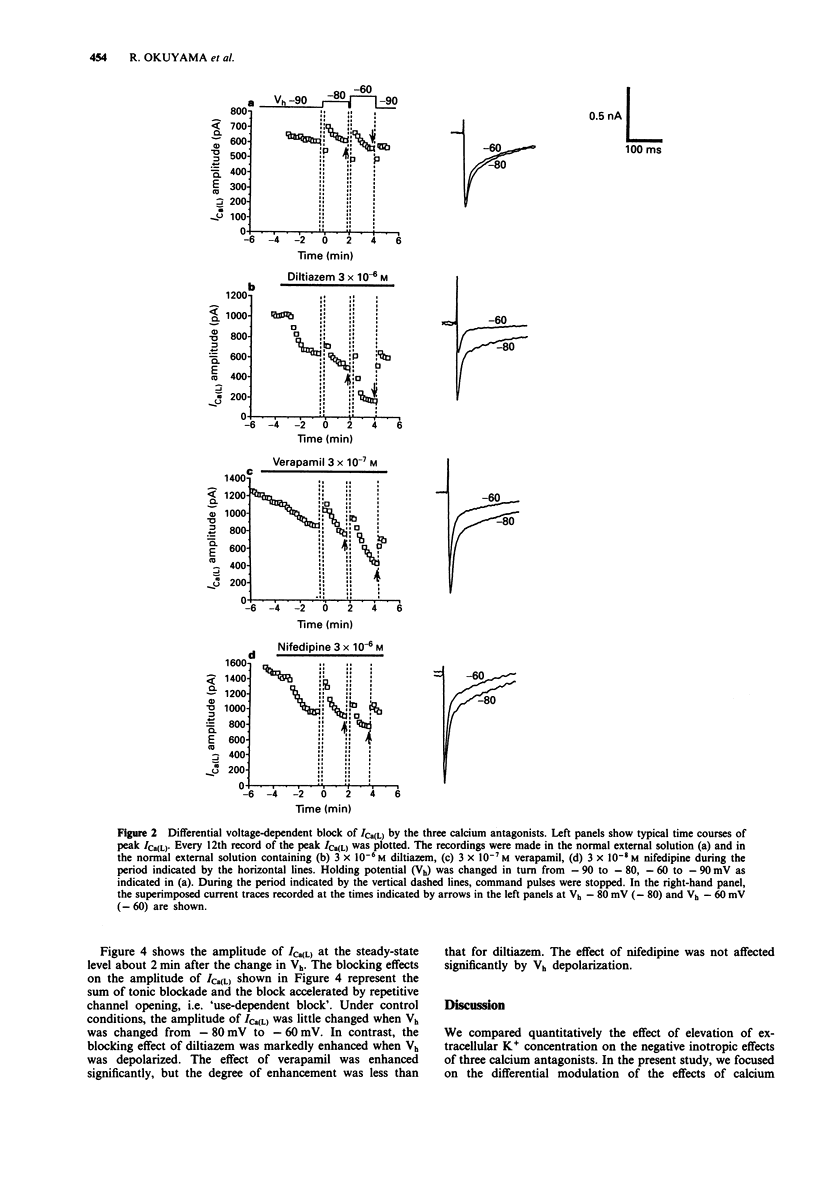
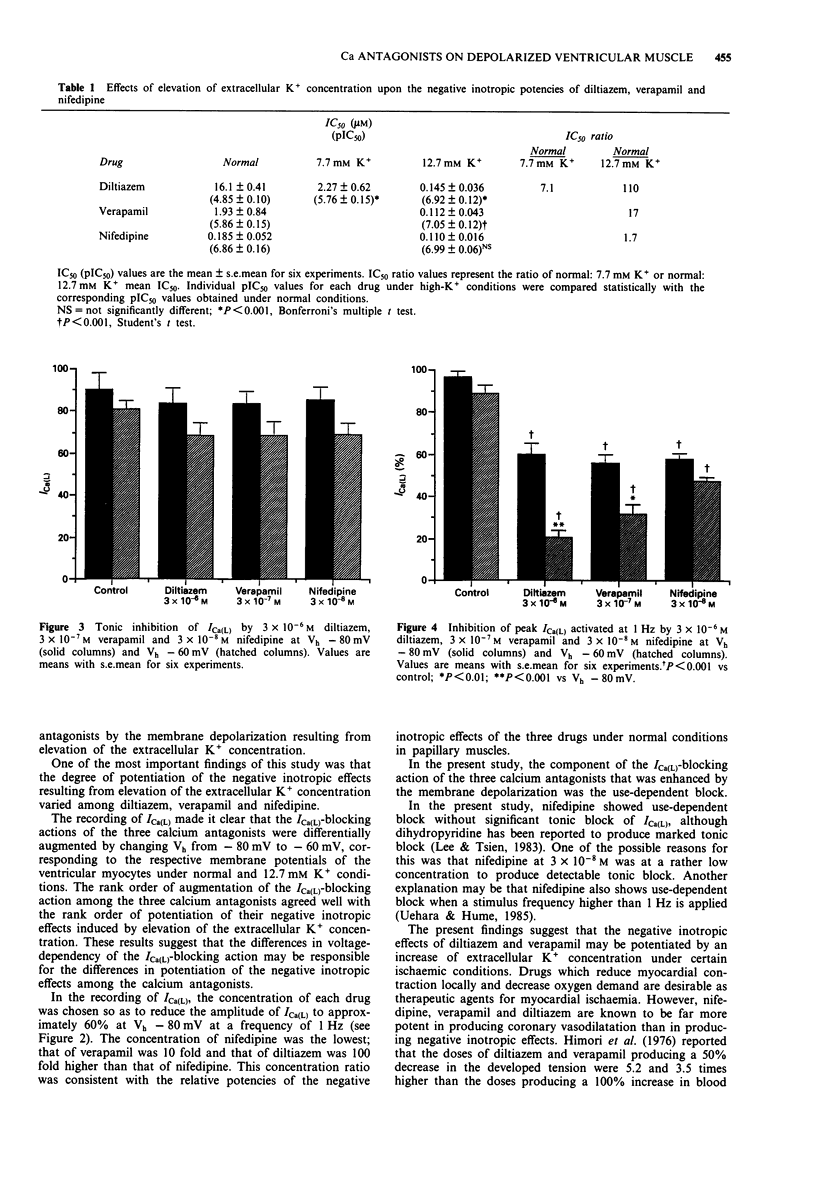
1. The effects of elevation of extracellular K+ concentration ([K+]o) on the negative inotropic potencies of three representative calcium antagonists, diltiazem, verapamil and nifedipine, were investigated in guinea-pig papillary muscle preparations. 2. The negative inotropic effect of diltiazem was potentiated 110 fold when [K+]o was raised from 2.7 mM to 12.7 mM. The effect of verapamil was also potentiated to a lesser extent, but that of nifedipine was not affected. 3. Resting membrane potentials in ventricular muscles were about -80 mV and -60 mV in 2.7 mM K+ and 12.7 mM K+, respectively. 4. To clarify the mechanism responsible for the differential potentiation of the negative inotropic effects, the blocking actions of the three calcium antagonists on the L-type Ca2+ channel current (ICa(L)) were compared at the holding potentials of -80 mV and -60 mV by the whole-cell patch-clamp technique. 5. The use-dependent blocking effect of diltiazem on ICa(L) was enhanced markedly by the change in the holding potential from -80 mV to -60 mV. The effect of verapamil was also enhanced to a lesser extent but that of nifedipine was not affected in this range of depolarization. 6. The differential effects of the [K+]o elevation on the negative inotropic potencies of the three calcium antagonists are explained by the differences in voltage-dependency of their use-dependent blocking effects on ICa(L). 7. The properties of diltiazem and verapamil observed in this study may contribute to their protective effects on the ischaemic myocardium, without affecting the normal myocardium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavalié A., Ochi R., Pelzer D., Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflugers Arch. 1983 Sep;398(4):284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Cox D. A., Matlib M. A. Modulation of intramitochondrial free Ca2+ concentration by antagonists of Na(+)-Ca2+ exchange. Trends Pharmacol Sci. 1993 Nov;14(11):408–413. doi: 10.1016/0165-6147(93)90063-P. [DOI] [PubMed] [Google Scholar]
- Ehara T., Daufmann R. The voltage- and time-dependent effects of (-)-verapamil on the slow inward current in isolated cat ventricular myocardium. J Pharmacol Exp Ther. 1978 Oct;207(1):49–55. [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Himori N., Ono H., Taira N. Simultaneous assessment of effects of coronary vasodilators on the coronary blood flow and the myocardial contractility by using the blood-perfused canine papillary muscle. Jpn J Pharmacol. 1976 Aug;26(4):427–435. doi: 10.1254/jjp.26.427. [DOI] [PubMed] [Google Scholar]
- Hirche H., Franz C., Bös L., Bissig R., Lang R., Schramm M. Myocardial extracellular K+ and H+ increase and noradrenaline release as possible cause of early arrhythmias following acute coronary artery occlusion in pigs. J Mol Cell Cardiol. 1980 Jun;12(6):579–593. doi: 10.1016/0022-2828(80)90016-4. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annu Rev Pharmacol Toxicol. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Abiko Y. Effect of diltiazem, a calcium antagonist, on myocardial pH in ischemic canine heart. J Pharmacol Exp Ther. 1982 Sep;222(3):720–725. [PubMed] [Google Scholar]
- Ichihara K., Sakai K., Abiko Y. Effect of nifedipine and niludipine on myocardial pH in the ischemic canine heart. Arch Int Pharmacodyn Ther. 1986 Mar;280(1):58–73. [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kanaya S., Katzung B. G. Effects of diltiazem on transmembrane potential and current of right ventricular papillary muscle of ferrets. J Pharmacol Exp Ther. 1984 Jan;228(1):245–251. [PubMed] [Google Scholar]
- Kloner R. A., Braunwald E. Effects of calcium antagonists on infarcting myocardium. Am J Cardiol. 1987 Jan 30;59(3):84B–94B. doi: 10.1016/0002-9149(87)90087-7. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Nagao T., Matlib M. A., Franklin D., Millard R. W., Schwartz A. Effects of diltiazem, a calcium antagonist, on regional myocardial function and mitochondria after brief coronary occlusion. J Mol Cell Cardiol. 1980 Jan;12(1):29–43. doi: 10.1016/0022-2828(80)90109-1. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Hoshiyama M., Yamashita K., Kiyomoto A. Electrical and mechanical responses to diltiazem in potassium depolarized myocardium of the guinea pig. Jpn J Pharmacol. 1976 Oct;26(5):571–580. doi: 10.1254/jjp.26.571. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Kikuchi Y., Senda Y., Yamada A., Koiwaya Y. Myocardial blood flow following experimental coronary occlusion. Effects of diltiazem. Chest. 1980 Jul;78(1 Suppl):205–209. [PubMed] [Google Scholar]
- Pérez J. E., Sobel B. E., Henry P. D. Improved performance of ischemic canine myocardium in response to nifedipine and diltiazem. Am J Physiol. 1980 Nov;239(5):H658–H663. doi: 10.1152/ajpheart.1980.239.5.H658. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Jennings R. B. Effects of calcium-channel blockers on myocardial preservation during experimental acute myocardial infarction. Am J Cardiol. 1985 Jan 25;55(3):107B–115B. doi: 10.1016/0002-9149(85)90619-8. [DOI] [PubMed] [Google Scholar]
- Taira N. Differences in cardiovascular profile among calcium antagonists. Am J Cardiol. 1987 Jan 30;59(3):24B–29B. doi: 10.1016/0002-9149(87)90078-6. [DOI] [PubMed] [Google Scholar]
- Triggle D. J. Calcium-channel drugs: structure-function relationships and selectivity of action. J Cardiovasc Pharmacol. 1991;18 (Suppl 10):S1–S6. [PubMed] [Google Scholar]
- Uehara A., Hume J. R. Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J Gen Physiol. 1985 May;85(5):621–647. doi: 10.1085/jgp.85.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand V., Güggi M., Meesmann W., Kessler M., Greitschus F. Extracellular potassium activity changes in the canine myocardium after acute coronary occlusion and the influence of beta-blockade. Cardiovasc Res. 1979 May;13(5):297–302. doi: 10.1093/cvr/13.5.297. [DOI] [PubMed] [Google Scholar]
- Zernig G. Widening potential for Ca2+ antagonists: non-L-type Ca2+ channel interaction. Trends Pharmacol Sci. 1990 Jan;11(1):38–44. doi: 10.1016/0165-6147(90)90040-f. [DOI] [PubMed] [Google Scholar]


