Abstract
Whole-body irradiation at the minimal lethal dose causes bone marrow failure and death within 12–18 days. To identify the principal components of the hematopoietic system that are radioprotective, we transplanted lethally irradiated mice with purified progenitors: common myeloid progenitors (CMPs), megakaryocyte/erythrocyte-restricted progenitors (MEPs), or granulocyte/monocyte-restricted progenitors (GMPs). Transplanted CMPs gave rise to cells both of the granulocyte/monocyte (GM) series and the megakaryocyte/erythrocyte series, whereas GMPs or MEPs showed reconstitution of only GM or ME cells, respectively. CMPs and MEPs but not GMPs protected mice in a dose-dependent manner, suggesting that erythrocytes, platelets, or both are the critical effectors of radioprotection. Accordingly, CMPs and MEPs formed robust colonies in recipient bone marrow and spleen, whereas GMPs formed small colonies that rapidly disappeared. Direct comparisons of spleen CFU (CFU-S) potentials among each progenitor subset showed that MEPs contain the vast majority of day 8 CFU-S activity, suggesting that day 8 CFU-S are the precursors of radioprotective cell subsets. All animals radioprotected for 30 days subsequently survived for at least 6 months post-transplant, and showed only host-derived hematopoiesis after 30 days. These findings suggest that rare hematopoietic stem cells survive myeloablation that can eventually repopulate irradiated hosts if myeloerythroid-restricted progenitors transiently rescue ablated animals through the critical window of bone marrow failure.
Introduction
Hematopoietic stem cells (HSCs) are clonogenic, self-renewing multipotent progenitors (MPPs) that generate all blood cell types to maintain hematopoiesis. Using assays for clonal precursors of myeloerythroid cells, B cells, and T cells, clonogenic mouse HSCs and MPPs were phenotypically defined and prospectively isolated (1, 2). These cells represent about one of every 2,000 bone marrow cells in young adult C57BL/Ka-Thy1.1 mice, and are about 2,000-fold enriched in the ability to radioprotect lethally irradiated congenic or syngeneic hosts (1, 2). Normally, lethally irradiated mice receiving no transplanted cells die of hematopoietic failure at 12–18 days. Mice protected by HSC transplants produce myeloerythroid cells but scant lymphoid cells by this time (3). Although these findings implicate myeloerythroid elements in radioprotection, it is not clear which myeloerythroid elements are responsible. Here we determine the in vivo activities of defined lineage-committed progenitors and test their roles in radioprotection.
The initial events in HSC differentiation include the transition of highly self-renewing long-term HSCs into short-term (ST) HSCs that possess limited self-renewal activity (2, 4). ST-HSCs subsequently generate MPPs that give rise to committed progenitors of various lineages (2, 5–11). The existence of HSCs and oligopotent progenitors within the hematopoietic system was initially shown by in vivo clonogenic assays. Till and McCulloch (12) discovered that mouse bone marrow contains highly proliferative progenitors capable of giving rise to colonies of hematopoietic cells within the spleens of lethally irradiated hosts. The colonies they analyzed at day 10 after transplant were composed of myeloerythroid cells, but a fraction of these colonies, when reinjected into mice, contained cells capable of long-term multilineage reconstitution that gave rise to both myeloid and lymphoid progeny (13, 14). Further characterization of spleen CFU (CFU-S)revealed that CFU-S at day 12 after transplant (day 12 CFU-S) derived from primitive MPPs and that day 8 CFU-S derived from relatively late myeloerythroid-committed progenitors (15).
The development of in vitro clonogenic assays has defined subsets of progenitors of the myeloid lineages that appear to have restricted differentiation capacity (16). Unfractionated bone marrow was found to contain oligopotent CFUs for all myeloid lineage cells (CFU-GEMM or CFU-Mix) (17, 18), for granulocytes and macrophages (CFU-GM)(19), and for megakaryocytes and erythrocytes (20). Monopotent CFUs for granulocytes, macrophages, erythrocytes (21), or megakaryocytes (22) were also found. We have recently described the prospective isolation of bone marrow progenitors that are the functional equivalents of the CFU-GEMM: common myeloid progenitors (CMPs), the CFU-GM (granulocyte/monocyte-restricted progenitors, or GMPs), and the CFU-ME (megakaryocyte/erythrocyte-restricted progenitors, or MEPs) (7). In vitro experiments testing progenitor/progeny relationships demonstrated that CMPs are clonogenic precursors of GMPs and MEPs, further substantiating the paradigm of progressive lineage restriction as HSCs differentiate into mature cell types (23).
Another kind of in vitro colony has been described that contains mainly large (blast) cells (24). Paired daughter cells derived from blast colonies frequently give rise to different combinations of myeloid progeny when split into identical conditions (25, 26). In similar experiments, paired progenitor assays using purified HSCs have also demonstrated such stochastic outcomes (27). These data collectively suggest that the lineages observed to derive from a single type of progenitor in vitro might not reflect the full commitment potential of each progenitor, and therefore, commitment models based on in vitro clonogenic assays may not correctly describe physiological differentiation processes in vivo. Here we determine the in vivo activities of CMPs, MEPs, and GMPs, and test their roles in radioprotection. We demonstrate that the vast majority of day 8 CFU-S are derived from MEPs, and that CMPs and MEPs but not GMPs protect mice from lethal-dose irradiation in a dose-dependent manner. These results indicate that day 8 CFU-S are comprised of erythrocyte/platelet precursors and that erythrocytes, platelets, or both are the critical effectors of radioprotection.
Methods
Mouse strains.
The congenic mouse strains C57BL/Ka-Thy1.1 (Ly5.1) and C57BL/Ka-Thy1.1-Ly5.2 were used as described (6). All animals were maintained in Stanford University’s Research Animal Facility in accordance with Stanford University guidelines.
Cell staining and sorting.
For myeloid progenitor experiments, bone marrow cells were stained with biotinylated antibodies specific for the following lineage markers: CD3 (KT31.1), CD4 (GK1.5), CD8 (53-6.7), B220 (6B2), Gr-1 (8C5), TER119, CD19 (1D3), IgM (R6-60.2; Pharmingen, San Diego, California, USA), Thy1.1 (19XE5), and IL-7Rα (A7R34). Cells positive for lineage markers (Lin+ cells) were partially removed with magnetic beads conjugated to sheep anti–rat IgG (Dynabeads M-450; Dynal AS, Oslo, Norway), and the remaining cells were stained with avidin-Cy5-phycoerythrin (PE) (Tricolor; Caltag Laboratories Inc., Burlingame, California, USA). Cells were stained with PE-conjugated anti-FcγR (2.4G2), FITC-conjugated CD34 (RAM34; Pharmingen), Texas red–conjugated anti–Sca-1 (E13-161-7), and allophycocyanin-conjugated (APC-conjugated) anti–c-Kit (2B8) monoclonal antibodies.
For sorting HSC and common lymphoid progenitor (CLP) populations, bone marrow cells were stained with unconjugated rat antibodies specific for Lin’s (CD3, CD4, CD8, B220, Mac-1, Gr-1, and TER119). Lin+ cells were partially removed with immunomagnetic beads conjugated to sheep anti–rat IgG, and the remaining cells were stained with Cy5-PE–conjugated goat anti–rat IgG polyclonal antibodies. After incubation with rat IgG (Sigma-Aldrich, St. Louis, Missouri, USA), cells were stained with FITC-conjugated anti–Thy-1.1 (19XE5), Texas red–conjugated anti–Sca-1, and APC-conjugated anti–c-Kit antibodies. IL-7Rα was stained with biotinylated anti–IL-7Rα antibody (A7R34) and was visualized with PE-conjugated streptavidin (Caltag Laboratories Inc.). All cell populations were sorted or analyzed using a highly modified triple laser (488-nm argon laser, 599-nm dye laser, and UV laser, FACS Vantage; Becton Dickinson Immunocytometry Systems, Mountain View, California, USA). Progenitors were purified by sorting and then re-sorting to obtain precise numbers of cells that were essentially pure for the indicated surface marker phenotype. In limiting dilution assays, the re-sort was performed using a carefully calibrated automatic cell deposition unit system (Becton Dickinson Immunocytometry Systems). This system deposited a specific number of purified cells onto OP9 stromal cell cultures in 96-well plates.
In vivo assays to determine differentiation potential of progenitors.
For reconstitution assays, purified progenitors were injected into the retroorbital venous sinus of lethally irradiated (9.5 Gy delivered in a split dose) congenic mice (differing only at the Ly5 allele) with or without 200 host-type HSCs. RAG2–/– recipients received 4 Gy. Intrathymic injections were performed by directly injecting cells into thymi of mice that had been irradiated (6 Gy) as described previously (6). CFU-S assays were performed with 100–500 double-sorted progenitors per mouse as previously described (28).
Peripheral blood analysis.
Peripheral blood was obtained from the tail vein of each mouse and collected into EDTA-containing tubes at the indicated time points. Complete blood counts were done using a CELL-DYN 3500 analyzer (Abbott Diagnostics, Mountain View, California, USA) calibrated for mouse blood samples. Flow cytometric analysis of granulocytes and monocytes was done as previously described (29). Donor-derived cells were distinguished from endogenous host cells by the expression of different Ly5 antigens (Ly5.1 vs. Ly5.2).
Results
Evaluation of in vivo colony-forming potentials of CMPs, MEPs, and GMPs.
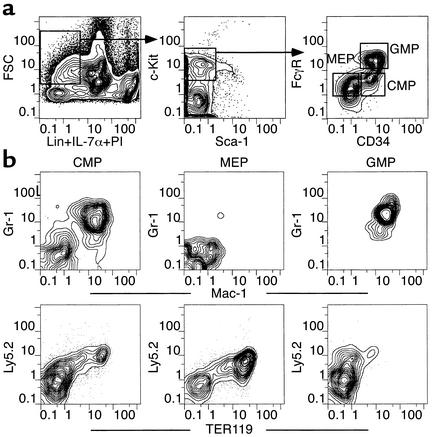
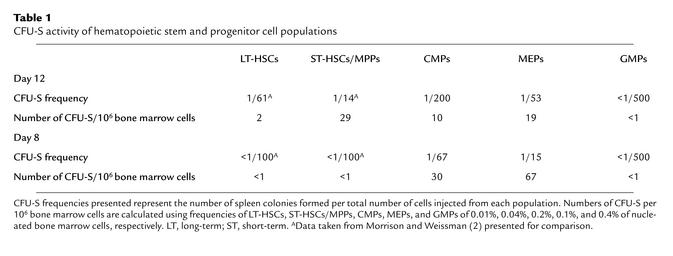
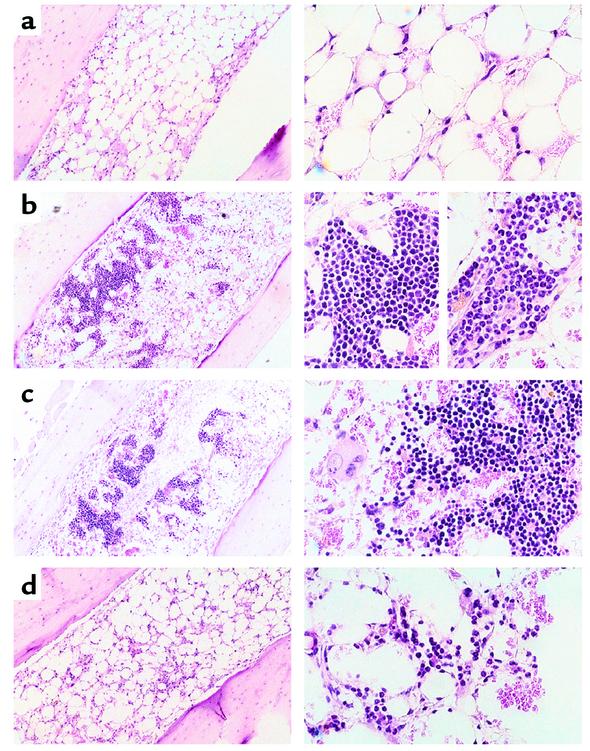
We first tested the activities of each myeloid progenitor subset in classical day 8 and day 12 CFU-S assays. Figure 1a shows the cell-surface phenotypes of CMPs, MEPs, and GMPs used in these studies. Parallel transplants comparing graded numbers of HSC and myeloid progenitor subsets showed that the majority of day 8 CFU-S activity resides within the MEP population (Table 1). The CMP population showed some day 8 CFU-S activity, while GMP cells had no detectable activity. On a per cell basis, some day 12 CFU-S derived from CMPs and MEPs, but the ST-HSC/MPP subsets were most highly enriched for day 12 CFU-S activity (1, 2). However, when the frequencies of these cell subsets were taken into account, about half of CFU-S day 12 activity in whole bone marrow resided within MEP/CMP populations and the other half within the HSC/MPP compartment. These data suggest that the majority of day 8 CFU-S are derived from MEPs, and are consistent with our previous observation that day 9 CFU-S were highly enriched in the Thy-1.1loLin–Sca-1– population (1). Accordingly, histological analyses of colonies within bone marrow sections showed that MEPs gave rise to large colonies composed of erythroid cells occasionally containing megakaryocytes but devoid of GM cells (Figure 2c). GMPs formed small diffuse colonies in bone marrow that contained only GM cells (Figure 2d). CMPs formed robust colonies in bone marrow that were composed of either ME or GM clusters (Figure 2b, left and right close-ups, respectively). In the spleen, CMPs and MEPs gave rise to colonies with similar appearances that appeared to be composed exclusively of erythroid precursors (not shown). GMPs rarely gave rise to spleen colonies; the few apparent colonies showed only diffuse clusters of macrophages.
Figure 1.
(a) Myeloid progenitor profiles in mouse bone marrow. Live Lin–IL-7Rα–Thy1.1– cells were gated and analyzed for expression of the c-Kit and Sca-1 surface markers. The IL-7Rα–Thy1.1–Lin–Sca-1–c-Kit+ fraction was subdivided into FcγRloCD34+(CMP), FcγRloCD34– (MEP), and FcγRhiCD34+ (GMP) populations. (b) In vivo differentiation potential of myeloid progenitors. Splenocytes from lethally irradiated congenic recipient mice were analyzed 6 days after intravenous injection of 10,000 CMPs, MEPs, or GMPs. Top row shows Gr-1/Mac-1 profiles of donor-derived (Ly5.2) cells in recipient mice (Ly5.1). Bottom row shows Ly5.2/TER119 profiles from the Gr-1–Mac-1– fractions shown above. FSC, forward scatter.
Table 1.
CFU-S activity of hematopoietic stem and progenitor cell populations
Figure 2.
Morphology of colonies derived from sorted CMPs, MEPs, and GMPs. Tibias were sectioned at day 7 after transplantation of (a) no cells, (b) 1 × 104 CMPs, (c) 6 × 103 MEPs, and (d) 2 × 104 GMPs into lethally irradiated recipients. Controls confirmed the absence of host-derived colonies. Left panels show field views at ×40 magnification, whereas right panels are close-up views at ×100 magnification. Erythroid colonies were generally larger and more compact (left close-up view in b) than were GM colonies (right close-up in b). Also note the distinct nuclear morphologies of granulocytes and monocytes in close-up colony views in b and d.
In vivo differentiation potentials of the three myeloid progenitor subsets.
We next tested the in vivo differentiation potential of these three progenitor populations. All three subsets quickly gave rise to mature cells in vivo, their progeny being detectable by 3–4 days after transplant (not shown). Six days after the injection of 10,000 CMPs into lethally irradiated recipient mice, both Gr-1+Mac-1+ myelomonocytic cells and TER119+ erythroid cells were detectable in the spleen (Figure 1b) and bone marrow (data not shown). In contrast, 10,000 GMPs transiently gave rise to only Gr-1+Mac-1+ cells, while equivalent MEP transplants transiently generated TER119+ cells but not GM cells. Thus, the lineage outcomes of each progenitor subset corresponded with those previously described in vitro (7).
Myeloerythroid progenitors do not have significant self-renewal activity.
To evaluate self-renewal potential and proliferative capacity, we injected 200 HSCs from C57BL/Ka-Thy1.1 (Ly5.1) and 5,000 cells of each myeloid progenitor population from C57BL/Ka-Thy1.1-Ly5.2 donors into lethally irradiated C57BL/6-Ly5.1 hosts. In this competitive reconstitution assay, the progeny from either FcγRloCD34– MEP or FcγRhiCD34+ GMP cells were undetectable after 2 weeks. The myeloid progeny from FcγRloCD34+ CMP cells were detectable at 2 weeks and 3 weeks (data not shown), but disappeared by 4 weeks after injection. This indicates that these populations have only transient repopulation potentials with limited or no self-renewal activity.
CMPs and MEPs alone provide radioprotection.
Given that CMPs, GMPs, and MEPs show the same differentiation potential in vivo as previously shown in vitro, we tested the capacity of these myeloid-restricted progenitors to radioprotect lethally irradiated congenic recipient mice without additional provision of HSCs or helper bone marrow. Previous experiments transplanting ST-HSCs/MPPs whose myeloid progenies are gone by 8 weeks suggested that early radioprotection allows rare surviving host HSCs to reach radioprotective levels after the 12- to 18-day critical window of bone marrow failure (2). To exclude the possibility of contamination by HSCs, double-sorted myeloid progenitor cells (>99% purity) were used for all transplants. While transplantation of GMPs alone provided no radioprotection, both CMP and MEP transplants showed dose-dependent rescue of irradiated recipients (Figure 3, a–c). Ten thousand CMPs or MEPs prolonged the survival of irradiated hosts to around day 21 but failed to ensure their survival after 30 days. However, when larger numbers of CMPs or MEPs were transplanted, we began to observe long-term survivors among the recipients. Sixty percent and seventy-two percent of mice transplanted with 50,000 CMPs or MEPs, respectively, survived more than 6 weeks. Peripheral blood samples of surviving recipients were analyzed at day 42 by flow cytometry. Although all recipients showed full hematologic recovery, no donor-derived myeloid cells were present in the peripheral blood at this time point (Figure 3d), indicating that the radioprotective effect was indeed mediated by poorly self-renewing progenitor cells that do not contribute to long-term hematopoiesis.
Figure 3.
Radioprotection of mice transplanted with myeloid progenitors. (a–c) Kaplan-Meier survival curves of lethally irradiated mice transplanted with no cells (control, rectangles), GMPs (filled squares), MEPs (open squares), or CMPs (triangles). All mice receiving no cells died by day 14 after irradiation, consistent with hematopoietic failure. Radioprotection was defined as survival beyond day 30. (a) No radioprotection was detected at the dose of 10,000 cells. (b) While 30,000 GMPs did not confer radioprotection, the same number of CMPs and MEPs protected 41.17% (7/17) and 35.29% (6/17) of mice from postradiation death, respectively. (c) More than 60% of mice can be saved by either 50,000 CMPs or 50,000 MEPs. (d) A representative flow cytometric analysis of peripheral blood from a recipient of 50,000 MEPs at day 42 after transplantation. All Gr-1+Mac-1+ cells are host-derived (Ly5.1).
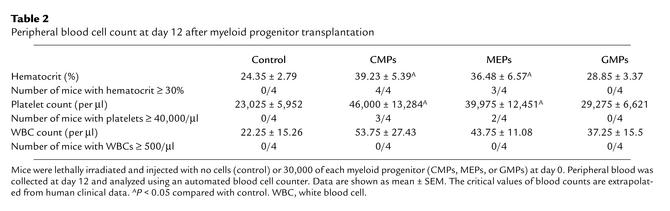
The nadir of blood counts after the minimum lethal dose of radiation is usually at day 15 when hematocrits of less than 15% and platelet counts below 20,000/μl are recorded (30). To evaluate the effect of myeloid progenitors on the level of circulating blood cells, we performed complete blood counts at day 12, before death from hematopoietic failure occurred. Mice injected with 30,000 MEPs or CMPs had significantly higher hematocrits and platelet counts than did radiation controls (Table 2). However, white blood cell counts were not significantly different between any of the groups. In fact, we never observed any recipients with more than 500 white blood cells/μl of blood (10% of normal value) at this time, even when up to 100,000 CMPs or GMPs were transplanted (data not shown). Taken together, these data strongly suggested that in these studies, erythrocytes and/or platelets are the critical effectors that prevent death during this period of bone marrow failure.
Table 2.
Peripheral blood cell count at day 12 after myeloid progenitor transplantation
Discussion
In this report, we functionally characterize three myeloerythroid-restricted progenitor subsets that have recently been isolated — CMPs and their lineal descendants, MEPs and GMPs (7). CMPs are downstream of HSCs, because CMPs do not give rise to HSCs, do not have self-renewal potential, and lack T cell differentiation potential (Figure 4). The identification of the CMPs, in addition to the previously reported CLPs (6), suggests that commitment to a myeloid or lymphoid lineage is a key developmental “choice” that occurs at distinct stages downstream of HSCs. The restricted capacity in vivo of CMPs and CLPs to differentiate into myeloid or lymphoid cells also suggests that significant plasticity between these lineages is rare once commitment occurs (31).
Figure 4.
Proposed model of murine hematopoiesis based on prospectively isolatable bone marrow populations. HSCs are long-term self-renewing cells that generate all blood cell types. IL-7Rα expression defines the commitment of MPPs to downstream CLPs that generate the lymphoid lineages. CMPs are clonogenic precursors of both GMPs and MEPs that respectively generate myelomonocytes and erythrocytes/platelets. Radioprotection capacity is present only in the highlighted populations that can generate erythrocytes and/or platelets in vivo.
The ability to prospectively isolate the progenitors representing the major branch points of the hematopoietic tree allows the functionalities of each subset to be tested in a precise manner. In this way, we examined each stem and progenitor subset for classical in vivo colony-forming and radioprotection activity. We found that the majority of day 8 CFU-S activity resides within the MEP population and is absent in GMPs, supporting previous findings that day 8–9 CFU-S are largely erythroid (1, 15, 32). CMPs presumably form splenic colonies by first giving rise to MEPs. Our data also reveal that about half of day 12 CFU-S activity resides within lineage-committed progenitors and about half within the more primitive HSC/MPP population (1, 2, 15). The day 12 activity observed within the MEP population may be explained by previous work suggesting that some day 12 colonies derive from surviving day 8 colonies (33). CLPs do not possess either day 8 or day 12 CFU-S activity (6). Thus, this study shows directly that the classical day 8 CFU-S assay largely measures MEP activity.
Radioprotection is defined as the ability to rapidly reconstitute an ablated blood-forming system in order to maintain viability of the host for a limited period of time, generally 30 days in the mouse. The populations responsible for this activity have been the subject of controversy (reviewed in ref. 34). While we have previously shown that as few as 100 HSCs can provide radioprotection (2, 3, 30), it has been argued that HSCs do not contribute directly to radioprotection since their relative quiescence causes significant delay of effector cell production (35, 36). Recently, other groups have also reported that long-term HSCs have poor radioprotective capacity and require the assistance of additional bone marrow subsets for full radioprotection (4, 10).
We tested the capacity of myeloid-restricted CMPs, MEPs, and GMPs to radioprotect lethally irradiated congenic recipient mice without additional provision of HSCs or helper bone marrow. While transplantation of GMPs alone provided no radioprotection, both CMP and MEP transplants showed dose-dependent rescue of irradiated recipients. The fact that CMPs and MEPs but not GMPs contain radioprotective capacity, together with the results of blood cell counts at day 12, strongly indicate that deficiencies of red blood cells, platelets, or both are normally responsible for mortality from hematopoietic failure following total body irradiation in mice. The hematocrits and platelet numbers 12 days after the injection of 30,000 MEPs or CMPs alone are comparable to the levels achieved by transplantation of a saturating dose of c-Kit+Thy-1.1loLin–/loSca-1+ (KTLS) HSCs (≥ 5,000 cells) (30). Interestingly, host-type cells gradually recovered in rescued recipients, and after 30 days constituted all blood cells. This is similar to transplants of radioprotective doses of ST-HSCs or MPPs (2), indicating that transplanted CMPs, MEPs, MPPs, and ST-HSCs provide transient production of erythrocytes and platelets sufficient to maintain host viability until rare, residual HSCs present in the host can take over. Therefore, in these studies, radioprotection is determined only by the capacity of cells to provide sufficient erythropoiesis and/or megakaryopoiesis during the period of bone marrow aplasia and is not the exclusive property of the HSC/MPP compartment. The requirement for high numbers of CMPs or MEPs to obtain significant radioprotection is probably due to their lack of self-renewal capacity, relatively short life spans, and small progeny burst sizes when compared with HSCs, which can replenish the CMP and MEP pools indefinitely.
Although the spleen does not normally contribute to human hematopoiesis, there are a number of conditions in which extensive splenic hematopoiesis can occur (37). This strongly indicates that human spleens contain a microenvironment capable of supporting proliferation and differentiation of hematopoietic stem and progenitor cells in vivo. Unfortunately, very few studies have been done to examine this aspect of hematopoietic recovery after bone marrow transplantation. It is generally agreed that macroscopic spleen colonies equivalent to mouse CFU-S do not exist in humans. However, microscopic bone marrow and spleen colonies can be found in a majority of patients shortly after bone marrow transplantation (38, 39). Interestingly, their characteristics are very similar to what we describe here for CMP- and MEP-derived colonies. Whether these colonies originate from the human counterparts of mouse CMPs and MEPs remains to be determined.
Cytopenia after total body irradiation or high-dose chemotherapy is one of the most serious problems in clinical oncology and transplantation. In our murine model, CMPs and MEPs significantly increase hematocrit and platelet counts in ablated recipients, whereas neutropenia cannot be prevented by transplantation of myeloid-restricted progenitors. Using a mathematical model simulating the clinical situation of peripheral blood transplantation, Scheding et al. have predicted that even transplantation of 1 × 107 CFU-GM/kg is not sufficient to avoid neutropenia after high-dose chemotherapy (40). We have transplanted high doses of GMPs — up to 100,000 per recipient (2 × 106/kg) — and never observed significant increases in blood neutrophil counts (data not shown). However, it is likely that neutropenia alone does not cause mortality unless serious bacterial or fungal infections occur. Therefore, the importance of myeloid progenitors to providing sufficient granulopoiesis could not be clearly demonstrated in this transplantation model because all irradiated recipients were kept on antibiotics under aseptic conditions. In another experimental system, we recently found that in conjunction with HSC transplants, the addition of CMP and GMP, but not MEP populations, protects mice from lethal infection by Aspergillus or Pseudomonas during this neutropenic period (A. Bitmansour et al., unpublished observations). Additionally, these progenitor populations are efficiently expanded and mobilized following standard protocols used in clinical transplantation and are all devoid of T lymphocyte differentiation potential in vivo (T. Na Nakorn, unpublished observations). Taken together, these results suggest that high-dose myeloid-restricted progenitor therapy might be effective in ameliorating the morbidity and mortality from myelosuppression caused by high-dose irradiation or chemotherapy regimens.
In summary, we have tested the behaviors of myeloerythroid-restricted progenitor subsets in a transplantation setting to model their normal physiological roles as closely as possible. All prospectively isolated myeloerythroid progenitor populations (CMPs, GMPs, and MEPs) showed transient reconstitution of irradiated hosts, and displayed fate outcomes in accordance with those of in vitro assays. Within the limits of the in vivo assays, they are non–self-renewing cells. CMPs and MEPs rapidly formed robust colonies in recipient marrow and spleen. Most importantly, CMPs and MEPs provided dose-dependent rescue of otherwise lethal irradiation. Our results also suggest that erythrocytes, platelets, or both are the critical effectors of radioprotection. The identification of counterpart populations in humans may allow us to test whether these progenitors can be used as adjuncts in hematopoietic cell transplantation to overcome some of the major complications seen in the clinic.
Acknowledgments
This research was supported by US Public Health Service grant CA-42551 (to I.L. Weissman) and by National Institute of Allergy and Infectious Diseases Training Grant 5T32 AI-07290 (to D. Traver). T. Na Nakorn was supported by the Anandamahidol Foundation under the royal patronage of His Majesty the King of Thailand. K. Akashi was supported by a 1997 Jose Carreras International Leukemia Foundation Grant and the 2000 Claudia Adams-Barr program. We thank S.-I. Nishikawa for the anti–IL-7Rα antibody, Amy Kiger for critical evaluation of the manuscript, Libuse Jerabek for excellent laboratory management and assistance with animal procedures, Veronica Braunstein for antibody preparation, the Stanford Shared FACS Facility for flow cytometer maintenance, and Lucino Hidalgo, Diosdado Escoto, and Bert Lavarro for animal care.
Footnotes
See the related Commentary beginning on page 1527.
David Traver’s present address is: Children’s Hospital, Harvard University, Boston, Massachusetts, USA.
Thanyaphong Na Nakorn and David Traver contributed equally to this work.
References
- 1.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 2.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 3.Uchida N, Aguila HL, Fleming WH, Jerabek L, Weissman IL. Rapid and sustained hematopoietic recovery in lethally irradiated mice transplanted with purified Thy-1.1loLin–Sca-1+hematopoietic stem cells. Blood. 1994;83:3758–3779. [PubMed] [Google Scholar]
- 4.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 5.Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 6.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 7.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 8.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long term stem cells. Proc Natl Acad Sci USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randall TD, Lund FE, Howard MC, Weissman IL. Expression of murine CD38 defines a population of long-term reconstituting hematopoietic stem cells. Blood. 1996;87:4057–4067. [PubMed] [Google Scholar]
- 10.Zhao Y, et al. Murine hematopoietic stem cell characterization and its regulation in BM transplantation. Blood. 2000;96:3016–3022. [PubMed] [Google Scholar]
- 11.Adolfsson J, et al. Upregulation of Flt3 expression within the bone marrow Lin-Sca1+c-kit+ stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 12.Till JE, McCulloch EA. A direct measure of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 13.Siminovitch L, McCulloch EA, Till JE. The distribution of colony-forming cells among spleen colonies. J Cell Comp Physiol. 1963;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 14.Lepault F, Ezine S, Gagnerault MC. T- and B-lymphocyte differentiation potentials of spleen colony-forming cells. Blood. 1993;81:950–955. [PubMed] [Google Scholar]
- 15.Magli MC, Iscove NN, Odartchenko N. Transient nature of early haematopoietic spleen colonies. Nature. 1982;295:527–529. doi: 10.1038/295527a0. [DOI] [PubMed] [Google Scholar]
- 16.Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44:287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 17.Johnson GR, Metcalf D. Pure and mixed erythroid colony formation in vitrostimulated by spleen conditioned medium with no detectable erythropoietin. Proc Natl Acad Sci USA. 1977;74:3879–3882. doi: 10.1073/pnas.74.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metcalf D, Johnson GR, Mandel TE. Colony formation in agar by multipotential hemopoietic cells. J Cell Physiol. 1979;98:401–420. doi: 10.1002/jcp.1040980216. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa Y, Pluznik DH, Sachs L. In vitrocontrol of the development of macrophage and granulocyte colonies. Proc Natl Acad Sci USA. 1966;56:488–495. doi: 10.1073/pnas.56.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLeod DL, Shreve MM, Axelrad AA. Induction of megakaryocyte colonies with platelet formation in vitro. Nature. 1976;261:492–494. doi: 10.1038/261492a0. [DOI] [PubMed] [Google Scholar]
- 21.Stephenson JR, Axelrad AA, McLeod DL, Shreeve MM. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci USA. 1971;68:1542–1546. doi: 10.1073/pnas.68.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metcalf D, MacDonald HR, Odartchenko N, Sordat B. Growth of mouse megakaryocyte colonies in vitro. Proc Natl Acad Sci USA. 1975;72:1744–1748. doi: 10.1073/pnas.72.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dexter TM. Introduction to the haemopoietic system. Cancer Surv. 1990;9:1–5. [PubMed] [Google Scholar]
- 24.Nakahata T, Ogawa M. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc Natl Acad Sci USA. 1982;79:3843–3847. doi: 10.1073/pnas.79.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suda J, Suda T, Ogawa M. Analysis of differentiation of mouse hemopoietic stem cells in culture by sequential replating of paired progenitors. Blood. 1984;64:393–399. [PubMed] [Google Scholar]
- 26.Suda T, Suda J, Ogawa M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc Natl Acad Sci USA. 1984;81:2520–2524. doi: 10.1073/pnas.81.8.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakauchi H, Takano H, Ema H, Osawa M. Further characterization of CD34-low/negative mouse hematopoietic stem cells. Ann NY Acad Sci. 1999;872:57–66. doi: 10.1111/j.1749-6632.1999.tb08453.x. [DOI] [PubMed] [Google Scholar]
- 28.Traver D, Akashi K, Weissman IL, Lagasse E. Mice defective in two apoptosis pathways in the myeloid lineage develop acute myeloblastic leukemia. Immunity. 1998;9:47–57. doi: 10.1016/s1074-7613(00)80587-7. [DOI] [PubMed] [Google Scholar]
- 29.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 30.Uchida N, et al. High doses of purified stem cells cause early hematopoietic recovery in syngeneic and allogeneic hosts. J Clin Invest. 1998;101:961–966. doi: 10.1172/JCI1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo M, et al. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–386. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 32.Humphries RK, Jacky PB, Dill FJ, Eaves AC, Eaves CJ. CFU-S in individual erythroid colonies derived in vitro from adult mouse marrow. Nature. 1979;279:718–720. doi: 10.1038/279718a0. [DOI] [PubMed] [Google Scholar]
- 33.Wolf NS, Priestley GV. Kinetics of early and late spleen colony development. Exp Hematol. 1986;14:676–682. [PubMed] [Google Scholar]
- 34.Morrison SJ, Wright DE, Cheshier SH, Weissman IL. Hematopoietic stem cells: challenges to expectations. Curr Opin Immunol. 1997;9:216–221. doi: 10.1016/s0952-7915(97)80138-0. [DOI] [PubMed] [Google Scholar]
- 35.Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 36.Jones RJ, et al. Characterization of mouse lymphohematopoietic stem cells lacking spleen colony-forming activity. Blood. 1996;88:487–491. [PubMed] [Google Scholar]
- 37.Lampert, I.A. 1994. Pathology of the spleen. In Disorders of the spleen. A. Cuschieri and C.D. Forbes, editors. Blackwell Scientific Publications. Oxford, United Kingdom. 51–77.
- 38.Cline MJ, Gale RP, Golde DW. Discrete clusters of hematopoietic cells in the marrow cavity of man after bone marrow transplantation. Blood. 1977;50:709–712. [PubMed] [Google Scholar]
- 39.Antin JH, Weinberg DS, Rappeport JM. Evidence that pluripotent stem cells form splenic colonies in humans after marrow transplantation. Transplantation. 1985;39:102–105. [PubMed] [Google Scholar]
- 40.Scheding S, et al. How many myeloid post-progenitor cells have to be transplanted to completely abrogate neutropenia after peripheral blood progenitor cell transplantation? Results of a computer simulation. Exp Hematol. 1999;27:956–965. doi: 10.1016/s0301-472x(99)00026-0. [DOI] [PubMed] [Google Scholar]