Abstract
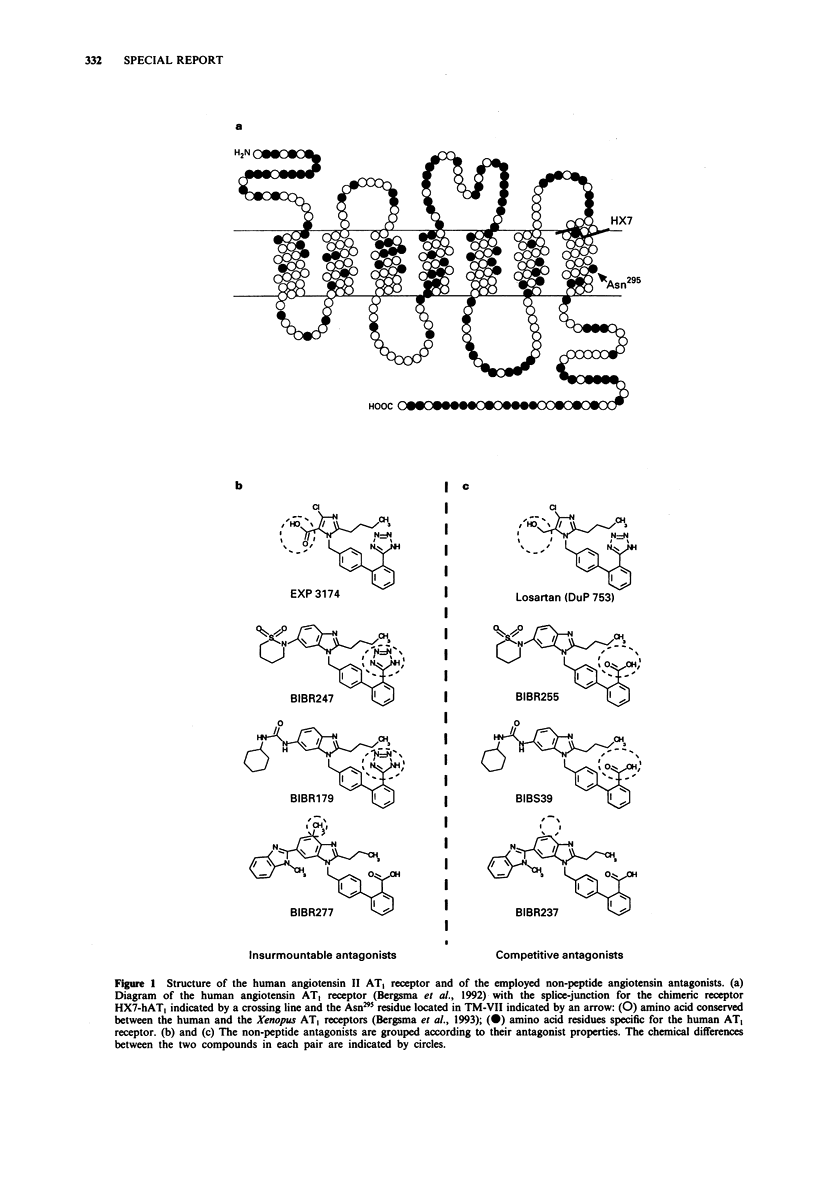
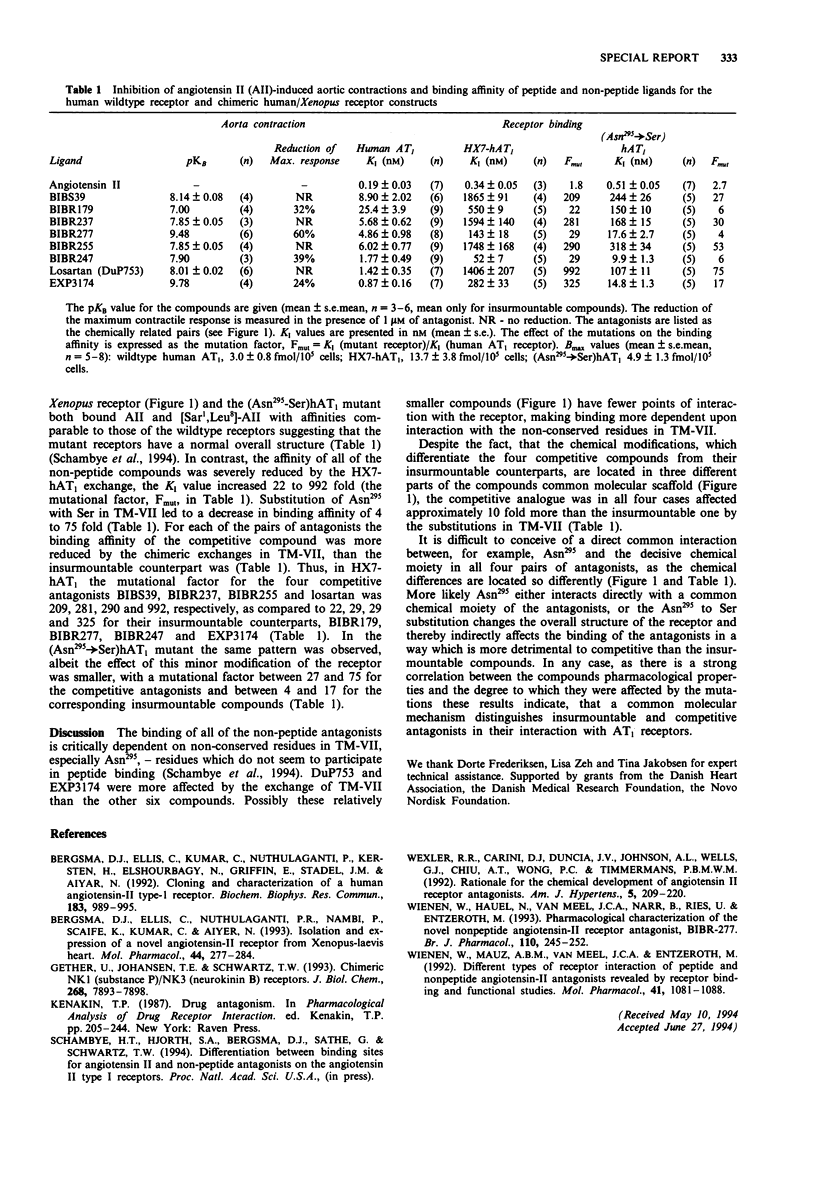
Chimeric constructs between the human and the Xenopus laevis AT1 receptor have demonstrated, that the binding of non-peptide angiotensin antagonists is dependent on non-conserved residues located deep in transmembrane segment VII of the AT1 receptor. Here we have studied four pairs of closely related antagonists each consisting of a competitive and an insurmountable compound differentiated by one out of three different types of minor chemical modifications. None of the antagonists bound to the Xenopus receptor and the binding of all of the compounds to the human receptor was severely impaired by the introduction of non-conserved residues from transmembrane segment VII of the Xenopus receptor. In all four pairs of antagonists the competitive compound was affected more by these substitutions than the corresponding insurmountable one (209 vs. 22, 281 vs. 29, 290 vs. 29 and 992 vs. 325-fold increase in Ki values). A similar pattern was observed in response to substitution of a single non-conserved residue in transmembrane segment VII, Asn295 to Ser. These results indicate that a common molecular mechanism distinguishes the interaction of insurmountable and competitive antagonists with the AT1 receptor.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsma D. J., Ellis C., Kumar C., Nuthulaganti P., Kersten H., Elshourbagy N., Griffin E., Stadel J. M., Aiyar N. Cloning and characterization of a human angiotensin II type 1 receptor. Biochem Biophys Res Commun. 1992 Mar 31;183(3):989–995. doi: 10.1016/s0006-291x(05)80288-8. [DOI] [PubMed] [Google Scholar]
- Bergsma D. J., Ellis C., Nuthulaganti P. R., Nambi P., Scaife K., Kumar C., Aiyar N. Isolation and expression of a novel angiotensin II receptor from Xenopus laevis heart. Mol Pharmacol. 1993 Aug;44(2):277–284. [PubMed] [Google Scholar]
- Gether U., Johansen T. E., Schwartz T. W. Chimeric NK1 (substance P)/NK3 (neurokinin B) receptors. Identification of domains determining the binding specificity of tachykinin agonists. J Biol Chem. 1993 Apr 15;268(11):7893–7898. [PubMed] [Google Scholar]
- Wienen W., Hauel N., Van Meel J. C., Narr B., Ries U., Entzeroth M. Pharmacological characterization of the novel nonpeptide angiotensin II receptor antagonist, BIBR 277. Br J Pharmacol. 1993 Sep;110(1):245–252. doi: 10.1111/j.1476-5381.1993.tb13800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienen W., Mauz A. B., Van Meel J. C., Entzeroth M. Different types of receptor interaction of peptide and nonpeptide angiotensin II antagonists revealed by receptor binding and functional studies. Mol Pharmacol. 1992 Jun;41(6):1081–1088. [PubMed] [Google Scholar]


