Abstract
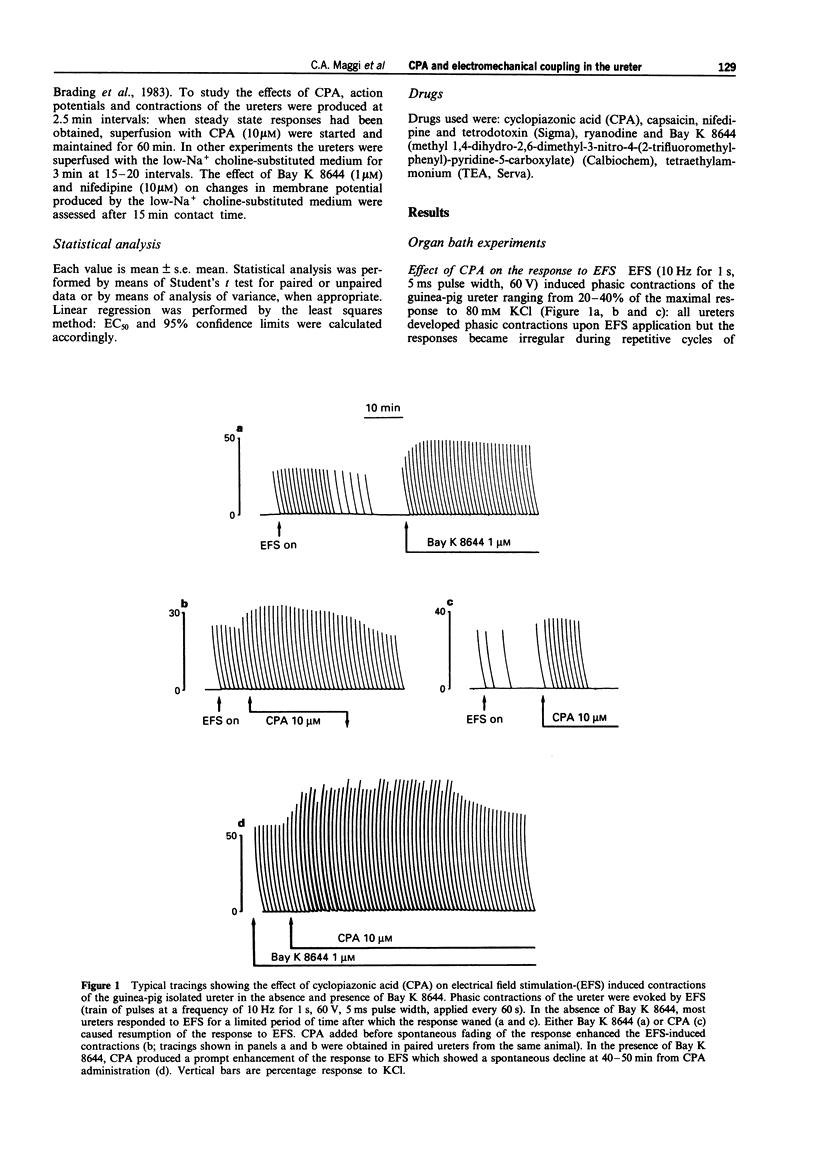
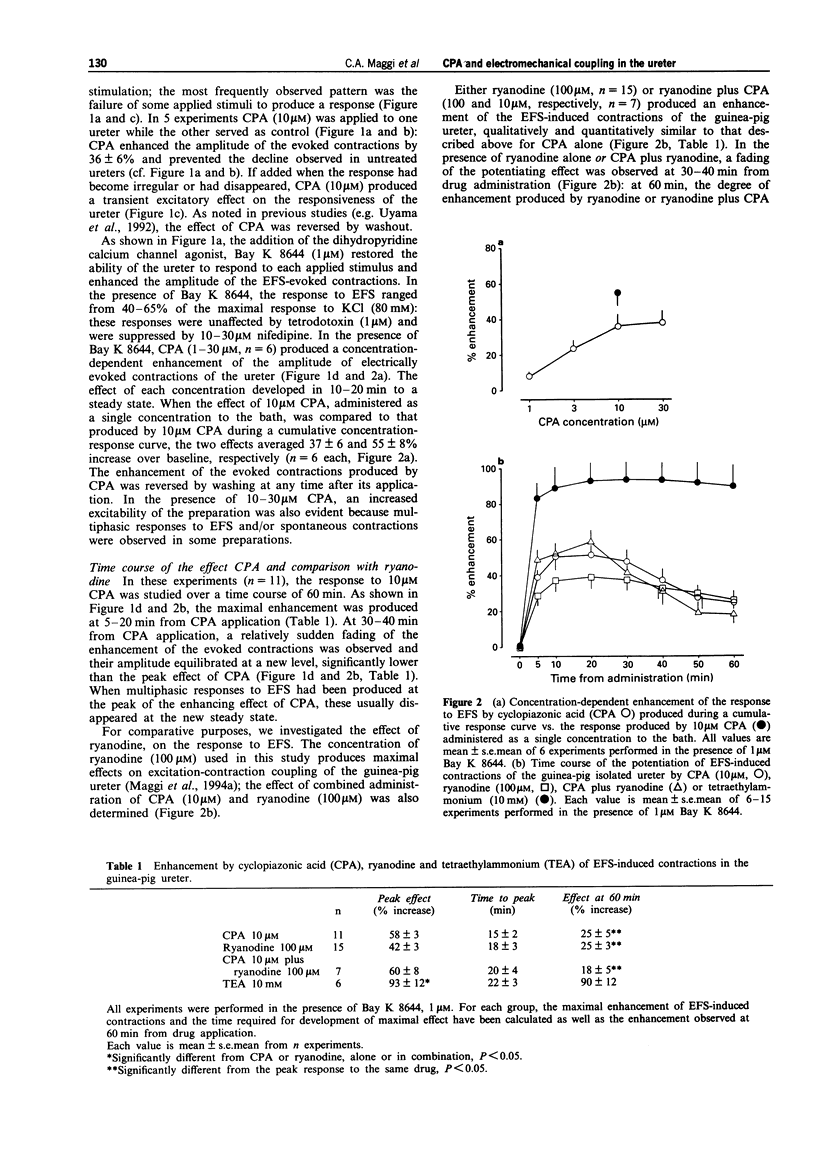
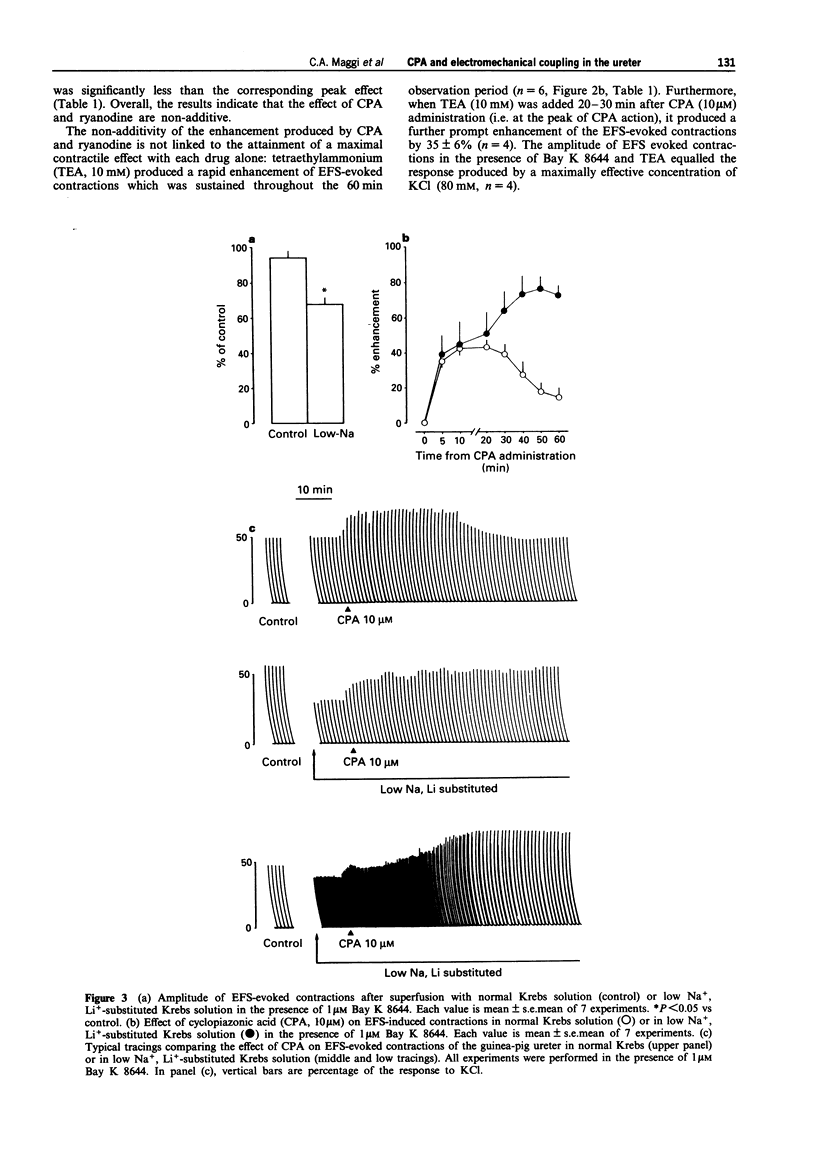
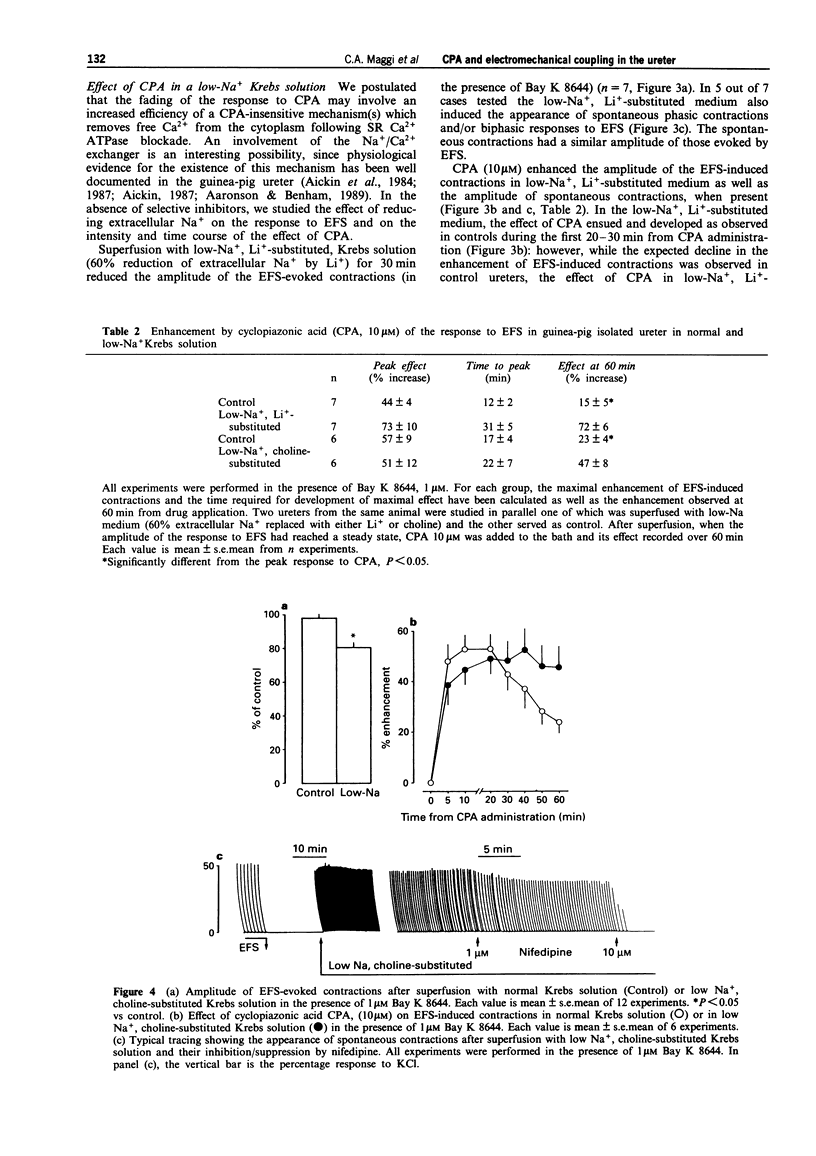
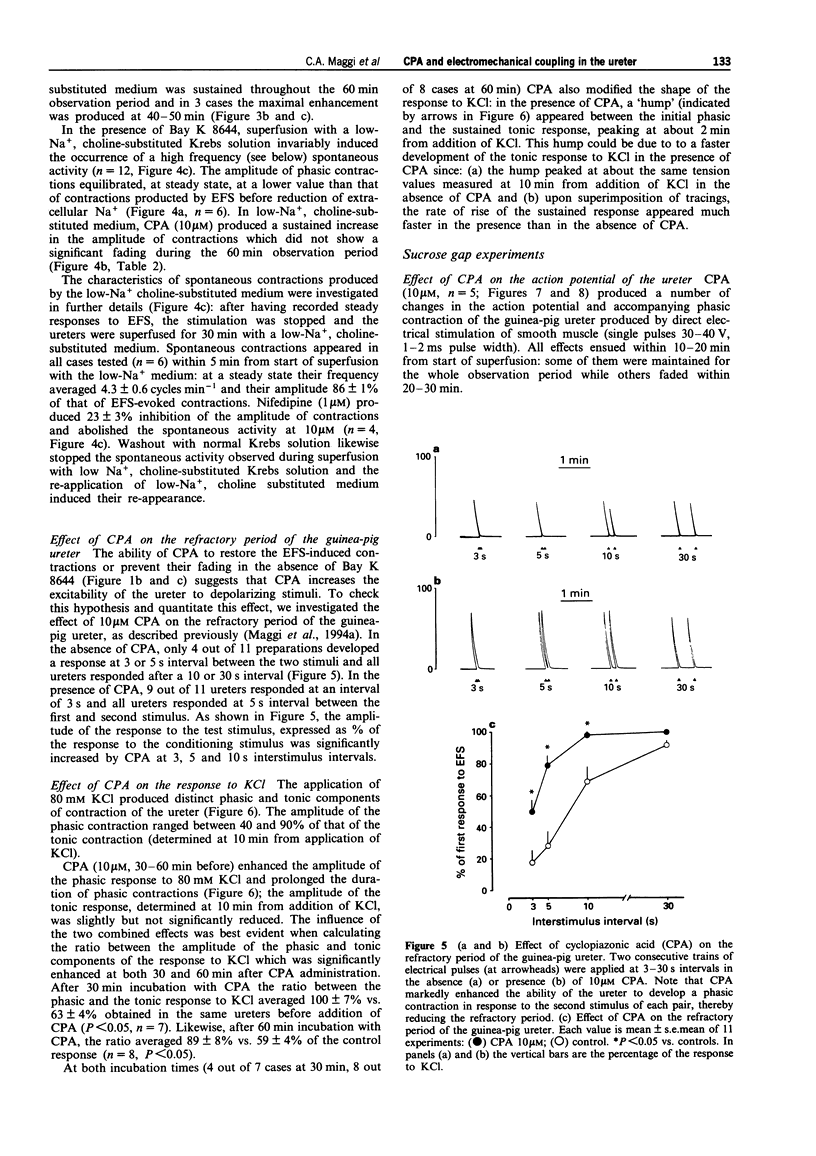
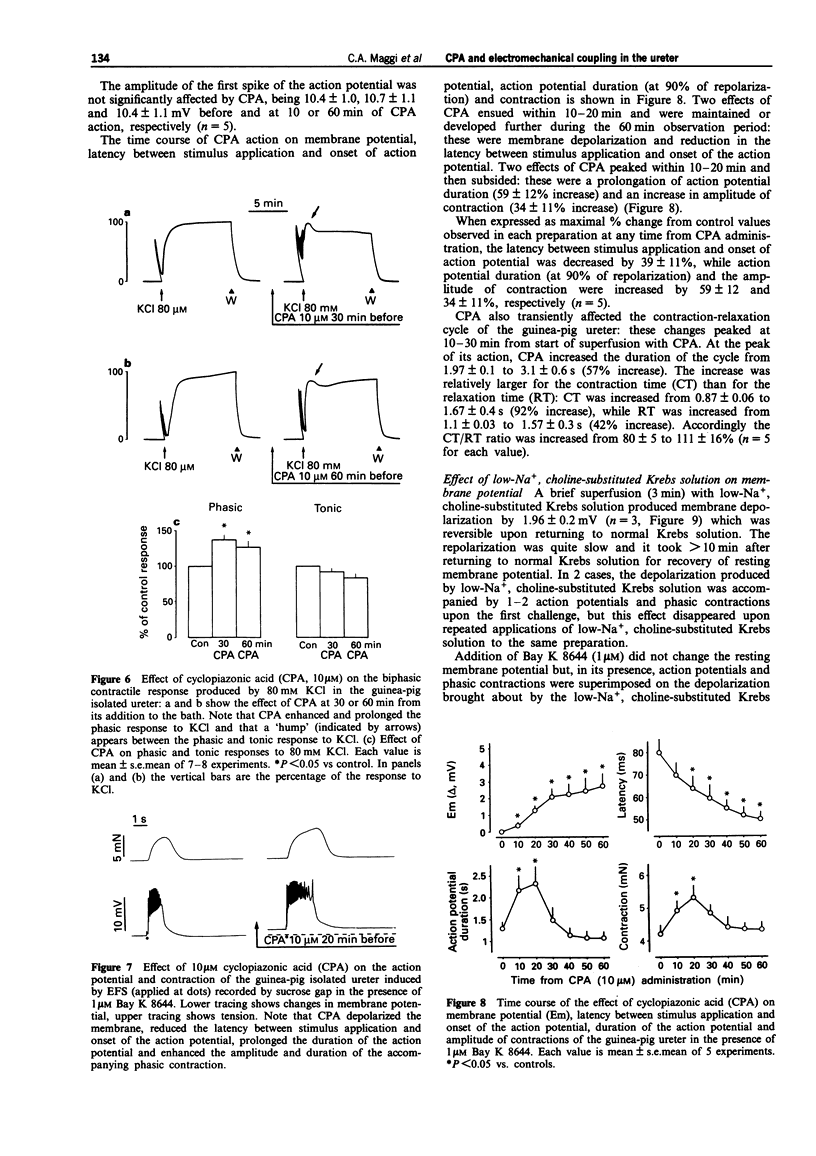
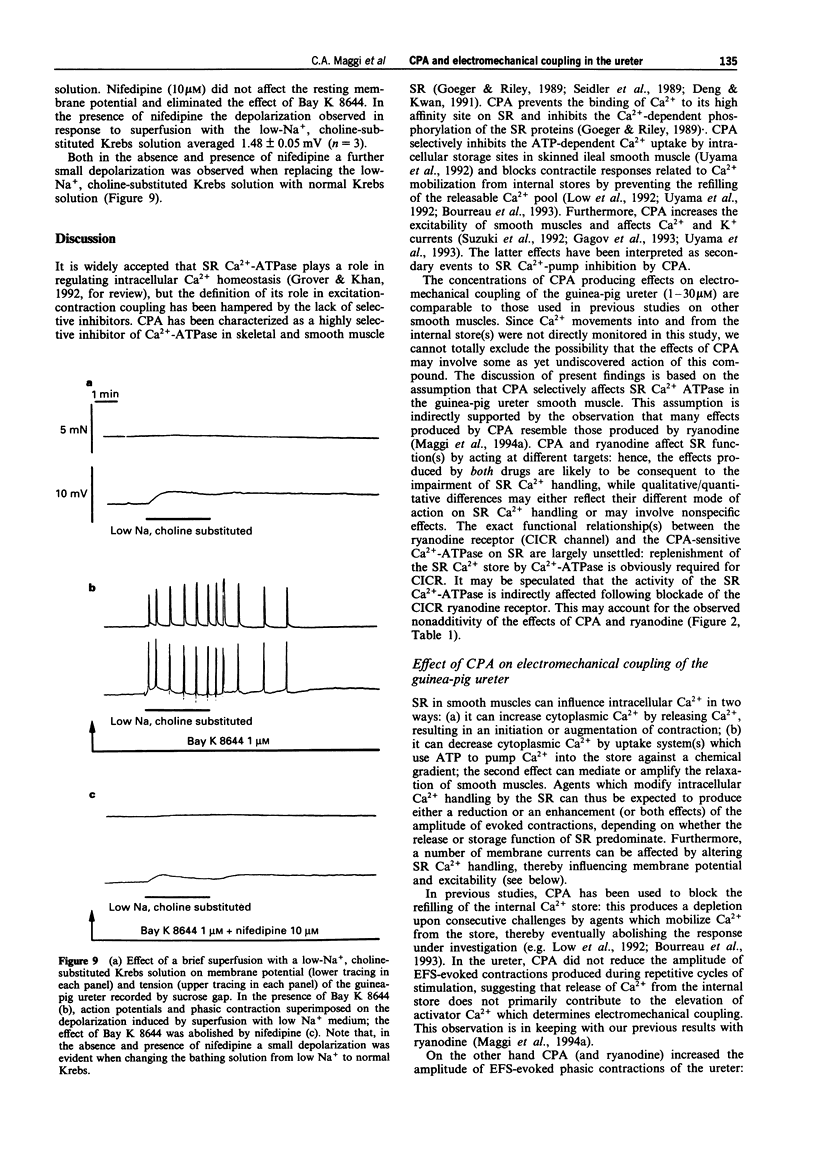
1. We have investigated the effect of the sarcoplasmic reticulum (SR) Ca(2+)-ATPase inhibitor, cyclopiazonic acid (CPA), on electromechanical coupling in the guinea-pig ureter. All experiments were performed in capsaicin-pretreated (10 microM for 15 min) ureters to prevent the release of sensory neuropeptides from afferent nerves. 2. In organ bath experiments, electrical field stimulation (EFS, 10 Hz for 1 s, 5 ms pulse width, 60 V) produced tetrodotoxin- (1 microM) resistant phasic contractions which were enhanced by Bay K 8644 (1 microM) and abolished by nifedipine (10-30 microM). 3. CPA (10 microM) enhanced the EFS-evoked contractions both in the absence and presence of Bay K 8644. The effect of CPA was concentration-dependent between 1 and 30 microM. The response to 10 microM CPA was biphasic: the maximal enhancement (58 +/- 3% increase) was observed within 10-20 min from CPA administration, followed by a decline to a new steady state (25 +/- 5% increase over baseline) at 50-60 min. The effect of CPA was reversed by washout. 4. Ryanodine (100 microM) produced a prompt enhancement of the EFS-evoked contractions of the guinea-pig ureter, which peaked at 42 +/- 3% increase over baseline; the co-administration of CPA (10 microM) and ryanodine (100 microM) produced a peak effect (60 +/- 8% enhancement) which was not different from that produced by CPA alone. With either ryanodine alone or ryanodine plus CPA, the enhancement of the EFS-induced contractions was biphasic, showing a time-course similar to that observed with CPA alone. Tetraethylammonium (10 mM) produced a significantly larger effect (93 +/- 13% increase over baseline) and its effect was sustained throughout the 60 min observation period. 5. In the presence of Bay K 8644, superfusion for 30 min with a low Na+ medium (60% of extracellular Na+ replaced by Li+ or choline) reduced the amplitude of EFS-evoked contractions by 20-35%. In both Li(+)- and choline-substituted media, spontaneous activity developed during superfusion with low Na+ Krebs solution which was suppressed by 10 microM nifedipine. CPA (10 microM) produced a marked enhancement of the EFS-evoked contractions in low-Na+ medium (both Li(+)- and choline-substituted) and this effect was sustained throughout the 60 min observation period.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P. I., Benham C. D. Alterations in [Ca2+]i mediated by sodium-calcium exchange in smooth muscle cells isolated from the guinea-pig ureter. J Physiol. 1989 Sep;416:1–18. doi: 10.1113/jphysiol.1989.sp017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F., Burdyga T. V. Evidence for sodium-calcium exchange in the guinea-pig ureter. J Physiol. 1984 Feb;347:411–430. doi: 10.1113/jphysiol.1984.sp015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F., Walmsley D. An investigation of sodium-calcium exchange in the smooth muscle of guinea-pig ureter. J Physiol. 1987 Oct;391:325–346. doi: 10.1113/jphysiol.1987.sp016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Investigation of factors affecting the intracellular sodium activity in the smooth muscle of guinea-pig ureter. J Physiol. 1987 Apr;385:483–505. doi: 10.1113/jphysiol.1987.sp016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artemenko D. P., Buryi V. A., Vladimirova I. A., Shuba M. F. Modifikatsiia metoda odinarnogo sakharoznogo mostika. Fiziol Zh. 1982 May;28(3):374–380. [PubMed] [Google Scholar]
- Bourreau J. P., Kwan C. Y., Daniel E. E. Distinct pathways to refill ACh-sensitive internal Ca2+ stores in canine airway smooth muscle. Am J Physiol. 1993 Jul;265(1 Pt 1):C28–C35. doi: 10.1152/ajpcell.1993.265.1.C28. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Burdyga T. V., Scripnyuk Z. D. The effects of papaverine on the electrical and mechanical activity of the guinea-pig ureter. J Physiol. 1983 Jan;334:79–89. doi: 10.1113/jphysiol.1983.sp014481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H. W., Kwan C. Y. Cyclopiazonic acid is a sarcoplasmic reticulum Ca(2+)-pump inhibitor of rat aortic muscle. Zhongguo Yao Li Xue Bao. 1991 Jan;12(1):53–58. [PubMed] [Google Scholar]
- Gagov H. S., Duridanova D. B., Boev K. K. Inhibition of Ca2+ current in ileal cells by cyclopiazonic acid and ryanodine. Eur J Pharmacol. 1993 Oct 12;243(1):19–24. doi: 10.1016/0014-2999(93)90162-b. [DOI] [PubMed] [Google Scholar]
- Goeger D. E., Riley R. T. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 1989 Nov 15;38(22):3995–4003. doi: 10.1016/0006-2952(89)90679-5. [DOI] [PubMed] [Google Scholar]
- Grover A. K., Khan I. Calcium pump isoforms: diversity, selectivity and plasticity. Review article. Cell Calcium. 1992 Jan;13(1):9–17. doi: 10.1016/0143-4160(92)90025-n. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H. A modified single sucrose gap. Junction potentials and electrotonic potentials in gastrointestinal smooth muscles. J Pharmacol Methods. 1987 Nov;18(3):219–226. doi: 10.1016/0160-5402(87)90072-6. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Takeda M., Watanabe M. Measurement and simulation of noninactivating Ca current in smooth muscle cells. Am J Physiol. 1989 Apr;256(4 Pt 1):C880–C885. doi: 10.1152/ajpcell.1989.256.4.C880. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Watanabe M. Ionic currents in single smooth muscle cells from the ureter of the guinea-pig. J Physiol. 1989 Apr;411:131–159. doi: 10.1113/jphysiol.1989.sp017565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Emptying and refilling of Ca2+ store in tracheal myocytes as indicated by ACh-evoked currents and contraction. Am J Physiol. 1993 Oct;265(4 Pt 1):C877–C886. doi: 10.1152/ajpcell.1993.265.4.C877. [DOI] [PubMed] [Google Scholar]
- Johnishi J., Sunano S. The role of membrane electrical activities and extracellular calcium in high-K-induced contracture of guinea pig ureter. Jpn J Physiol. 1978;28(1):1–16. doi: 10.2170/jjphysiol.28.1. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Tomita T. The action potential in the smooth muscle of the guinea pig taenia coli and ureter studied by the double sucrose-gap method. J Gen Physiol. 1970 Feb;55(2):147–162. doi: 10.1085/jgp.55.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. J. Identification of the major membrane currents in freshly dispersed single smooth muscle cells of guinea-pig ureter. J Physiol. 1989 May;412:375–395. doi: 10.1113/jphysiol.1989.sp017622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low A. M., Kwan C. Y., Daniel E. E. Evidence for two types of internal Ca2+ stores in canine mesenteric artery with different refilling mechanisms. Am J Physiol. 1992 Jan;262(1 Pt 2):H31–H37. doi: 10.1152/ajpheart.1992.262.1.H31. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S., Santicioli P. Effect of Bay K 8644 and ryanodine on the refractory period, action potential and mechanical response of the guinea-pig ureter to electrical stimulation. Naunyn Schmiedebergs Arch Pharmacol. 1994 May;349(5):510–522. doi: 10.1007/BF00169141. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S., Santicioli P. Effect of cromakalim and glibenclamide on spontaneous and evoked motility of the guinea-pig isolated renal pelvis and ureter. Br J Pharmacol. 1994 Mar;111(3):687–694. doi: 10.1111/j.1476-5381.1994.tb14792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S. The neurotransmitter role of calcitonin gene-related peptide in the rat and guinea-pig ureter: effect of a calcitonin gene-related peptide antagonist and species-related differences in the action of omega conotoxin on calcitonin gene-related peptide release from primary afferents. Neuroscience. 1991;43(1):261–268. doi: 10.1016/0306-4522(91)90433-o. [DOI] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Shuba M. F. The effect of sodium-free and potassium-free solutions, ionic current inhibitors and ouabain on electrophysiological properties of smooth muscle of guinea-pig ureter. J Physiol. 1977 Jan;264(3):837–851. doi: 10.1113/jphysiol.1977.sp011697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Muraki K., Imaizumi Y., Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca(2+)-pump, reduces Ca(2+)-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 1992 Sep;107(1):134–140. doi: 10.1111/j.1476-5381.1992.tb14475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama Y., Imaizumi Y., Watanabe M. Cyclopiazonic acid, an inhibitor of Ca(2+)-ATPase in sarcoplasmic reticulum, increases excitability in ileal smooth muscle. Br J Pharmacol. 1993 Oct;110(2):565–572. doi: 10.1111/j.1476-5381.1993.tb13848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama Y., Imaizumi Y., Watanabe M. Effects of cyclopiazonic acid, a novel Ca(2+)-ATPase inhibitor, on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 1992 May;106(1):208–214. doi: 10.1111/j.1476-5381.1992.tb14316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washizu Y. Membrane potential and tension in guinea-pig ureter. J Pharmacol Exp Ther. 1967 Dec;158(3):445–450. [PubMed] [Google Scholar]


