Abstract
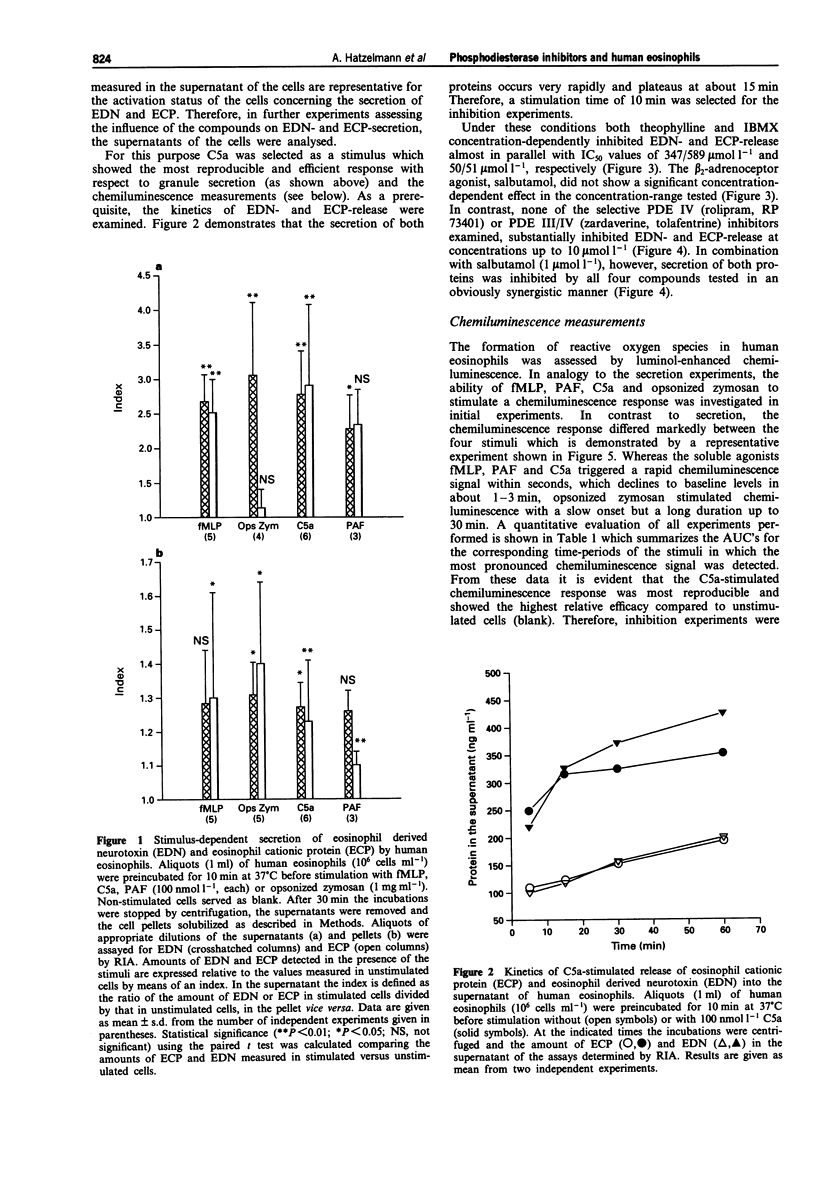
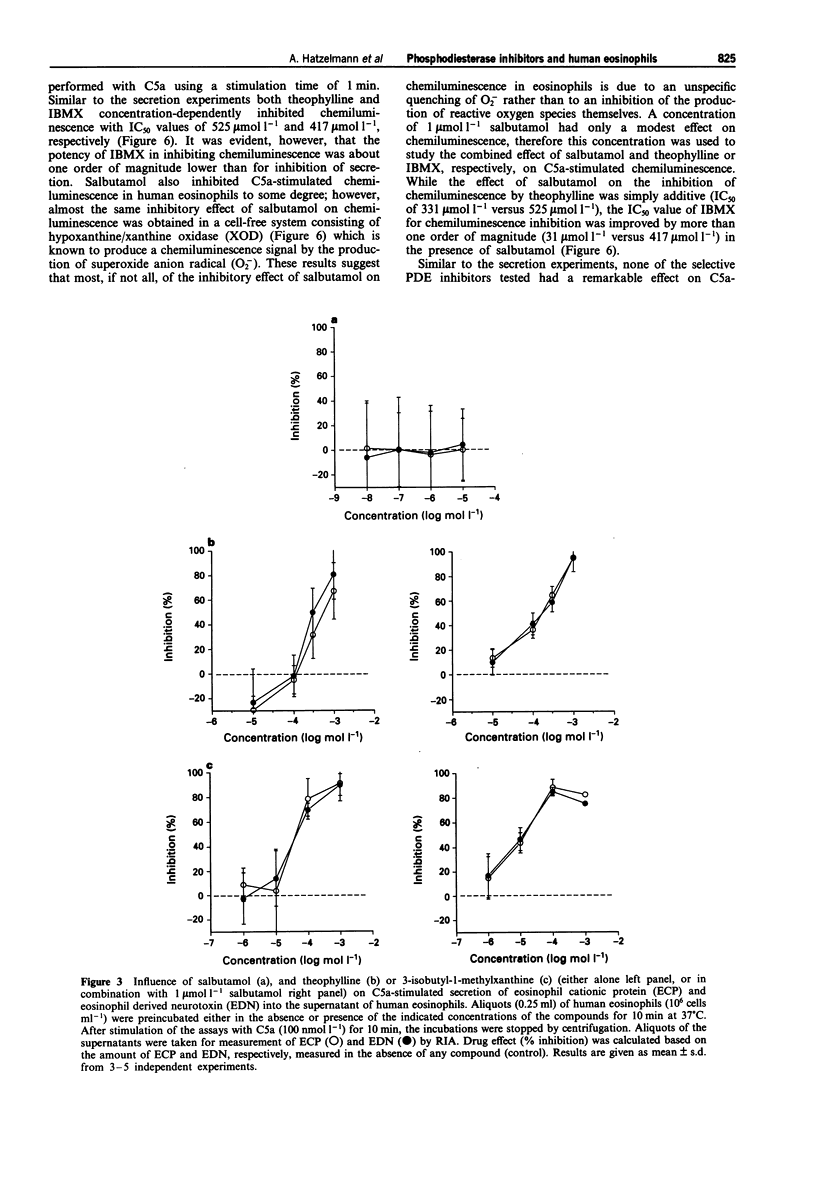
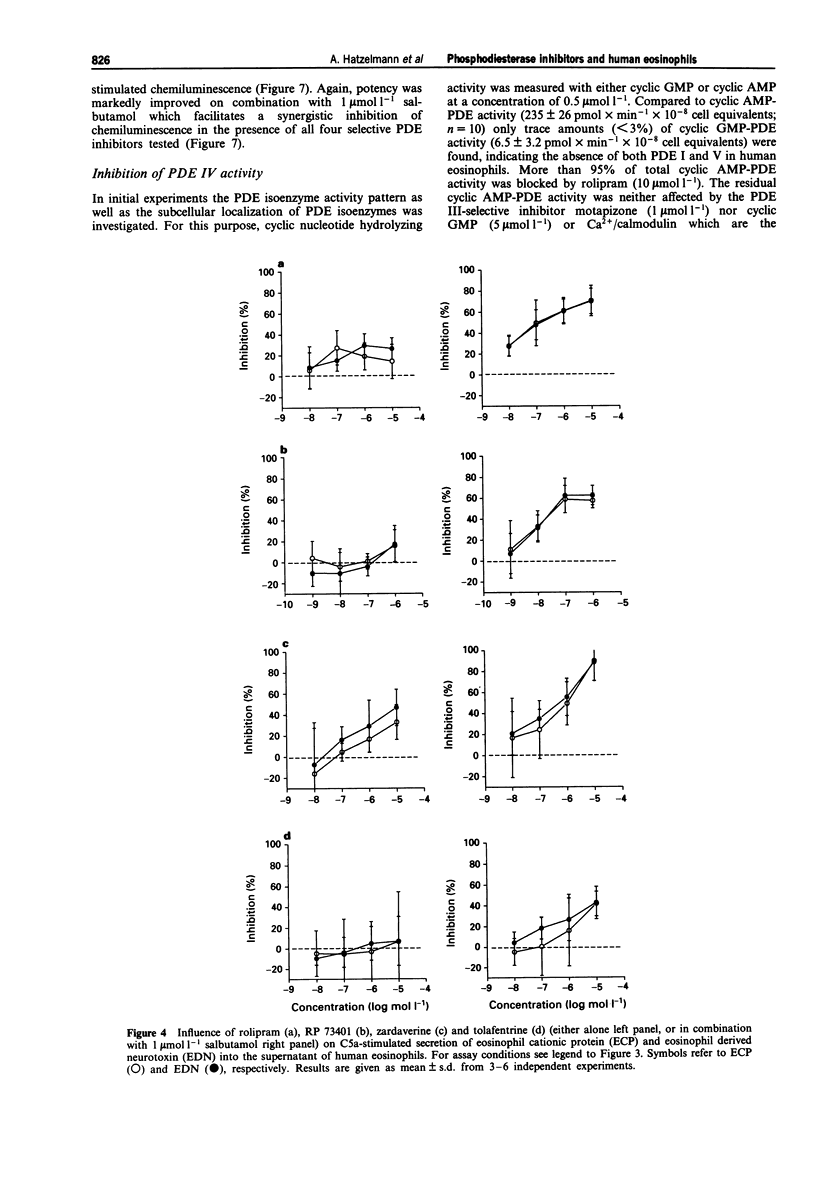
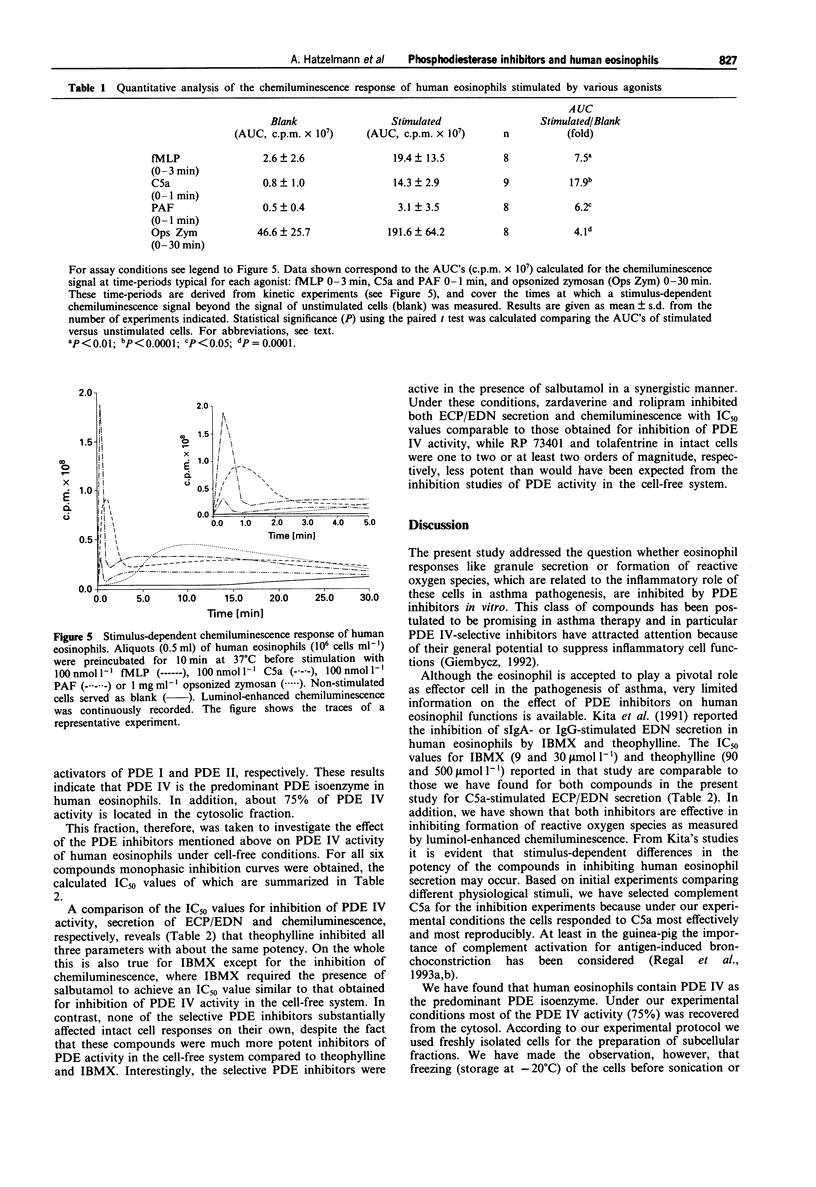
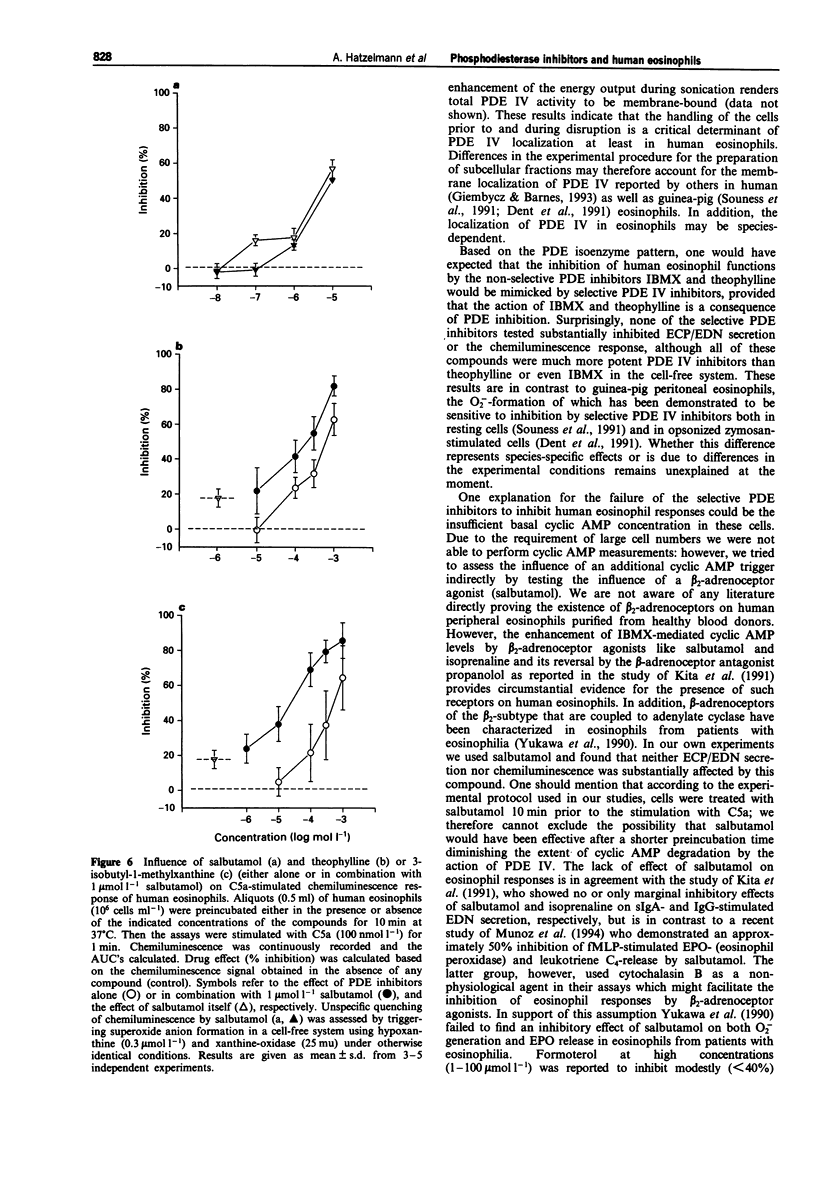
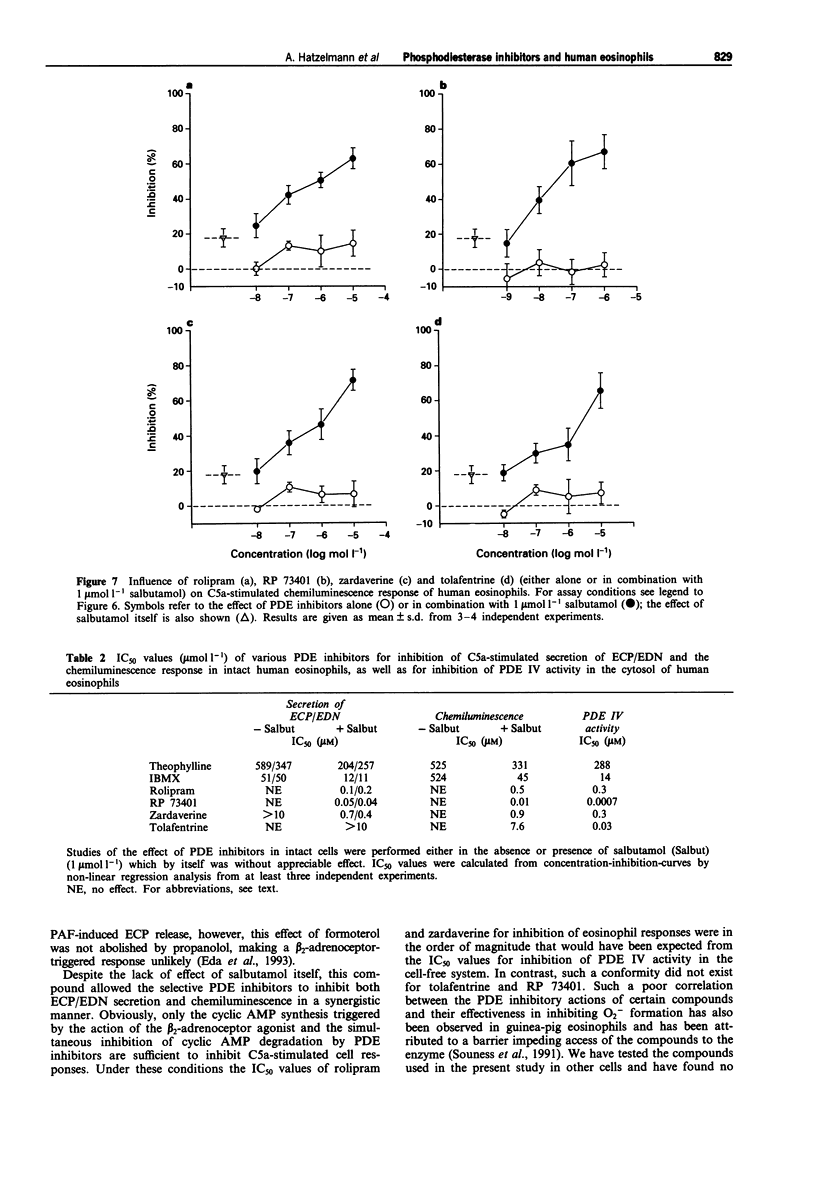
1. The effect of non-selective (3-isobutyl-1-methylxanthine, IBMX; theophylline) and type IV- or type III/IV-selective (rolipram, RP 73401; zardaverine, tolafentrine) phosphodiesterase (PDE) inhibitors on human eosinophil functions was investigated. 2. For this purpose human eosinophils were purified from blood of healthy donors by a magnetic cell separation (MACS) technique to a purity > or = 99%. From the stimuli investigated (complement C5a; N-formyl-methionyl-leucyl-phenylalanine, fMLP; platelet activating factor, PAF; opsonized zymosan) C5a was selected to test the influence of the above mentioned compounds on secretion of granule constituents (eosinophil cationic protein, ECP; eosinophil-derived neurotoxin, EDN) as well as on formation of reactive oxygen species measured by luminol-enhanced chemiluminescence in intact cells. For comparison, inhibition of PDE IV activity in the cytosol of disrupted cells, which contains about 75% of total PDE IV activity, was determined. 3. Both theophylline and IBMX inhibited the two cell responses with IC50 values which were in the range of their IC50 values obtained for inhibition of PDE IV activity in the cell-free system. The beta 2-adrenoceptor agonist, salbutamol (1 mumol l-1), which by itself did not substantially influence the two cell responses, only marginally improved the potency of theophylline and IBMX in inhibiting ECP/EDN secretion. Only the IC50 value of IBMX for inhibition of chemiluminescence was lowered by about one order of magnitude in the presence of salbutamol. 4. In contrast, none of the selective PDE inhibitors tested substantially inhibited the two cell responses at concentrations up to 10 mumol l-1. This was surprising because all of the compounds investigated inhibited PDE IV activity in the cell-free system with IC50 values which were at least 30 fold lower than the highest concentration of the compounds used with intact cells. In combination with salbutamol, however, both ECP/EDN secretion and chemiluminescence was inhibited by rolipram and zardaverine with IC50 values similar to the IC50 values for inhibition of PDE IV activity. Although RP 73401 and tolafentrine also inhibited both cell responses in the presence of salbutamol, the potency of these two compounds in inhibiting eosinophil function in intact cells was at least two orders of magnitude lower than would have been expected from the inhibition of PDE IV activity in the cell-free system.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Ghazaleh R. I., Dunnette S. L., Loegering D. A., Checkel J. L., Kita H., Thomas L. L., Gleich G. J. Eosinophil granule proteins in peripheral blood granulocytes. J Leukoc Biol. 1992 Dec;52(6):611–618. doi: 10.1002/jlb.52.6.611. [DOI] [PubMed] [Google Scholar]
- Bach M. K., Brashler J. R., Sanders M. E., Bienkowski M. J. Pitfalls in the quantitative estimation of the secretion of granule proteins by eosinophils. J Immunol Methods. 1991 Sep 13;142(2):243–250. doi: 10.1016/0022-1759(91)90112-s. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. New aspects of asthma. J Intern Med. 1992 May;231(5):453–461. doi: 10.1111/j.1365-2796.1992.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Pedersen S. Efficacy and safety of inhaled corticosteroids in asthma. Report of a workshop held in Eze, France, October 1992. Am Rev Respir Dis. 1993 Oct;148(4 Pt 2):S1–26. doi: 10.1164/ajrccm/148.4_Pt_2.S1. [DOI] [PubMed] [Google Scholar]
- Bauer A. C., Schwabe U. An improved assay of cyclic 3',5'-nucleotide phosphodiesterases with QAE-Sephadex columns. Naunyn Schmiedebergs Arch Pharmacol. 1980 Mar;311(2):193–198. doi: 10.1007/BF00510259. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel P. L. Contribution of eosinophil-derived mediators in asthma. Int Arch Allergy Appl Immunol. 1989;90 (Suppl 1):57–63. doi: 10.1159/000235077. [DOI] [PubMed] [Google Scholar]
- Dent G., Giembycz M. A., Rabe K. F., Barnes P. J. Inhibition of eosinophil cyclic nucleotide PDE activity and opsonised zymosan-stimulated respiratory burst by 'type IV'-selective PDE inhibitors. Br J Pharmacol. 1991 Jun;103(2):1339–1346. doi: 10.1111/j.1476-5381.1991.tb09790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eda R., Sugiyama H., Hopp R. J., Okada C., Bewtra A. K., Townley R. G. Inhibitory effects of formoterol on platelet-activating factor induced eosinophil chemotaxis and degranulation. Int Arch Allergy Immunol. 1993;102(4):391–398. doi: 10.1159/000236588. [DOI] [PubMed] [Google Scholar]
- Giembycz M. A. Could isoenzyme-selective phosphodiesterase inhibitors render bronchodilator therapy redundant in the treatment of bronchial asthma? Biochem Pharmacol. 1992 May 28;43(10):2041–2051. doi: 10.1016/0006-2952(92)90160-k. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Adolphson C. R. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- Gleich G. J. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990 Feb;85(2):422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Hansel T. T., De Vries I. J., Iff T., Rihs S., Wandzilak M., Betz S., Blaser K., Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991 Dec 15;145(1-2):105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- Kita H., Abu-Ghazaleh R. I., Gleich G. J., Abraham R. T. Regulation of Ig-induced eosinophil degranulation by adenosine 3',5'-cyclic monophosphate. J Immunol. 1991 Apr 15;146(8):2712–2718. [PubMed] [Google Scholar]
- Michaeli T., Bloom T. J., Martins T., Loughney K., Ferguson K., Riggs M., Rodgers L., Beavo J. A., Wigler M. Isolation and characterization of a previously undetected human cAMP phosphodiesterase by complementation of cAMP phosphodiesterase-deficient Saccharomyces cerevisiae. J Biol Chem. 1993 Jun 15;268(17):12925–12932. [PubMed] [Google Scholar]
- Milgrom H., Bender B. Current issues in the use of theophylline. Am Rev Respir Dis. 1993 Jun;147(6 Pt 2):S33–S39. doi: 10.1164/ajrccm/147.6_Pt_2.S33. [DOI] [PubMed] [Google Scholar]
- Munoz N. M., Vita A. J., Neeley S. P., McAllister K., Spaethe S. M., White S. R., Leff A. R. Beta adrenergic modulation of formyl-methionine-leucine-phenylalanine-stimulated secretion of eosinophil peroxidase and leukotriene C4. J Pharmacol Exp Ther. 1994 Jan;268(1):139–143. [PubMed] [Google Scholar]
- Parsons W. J., Ramkumar V., Stiles G. L. Isobutylmethylxanthine stimulates adenylate cyclase by blocking the inhibitory regulatory protein, Gi. Mol Pharmacol. 1988 Jul;34(1):37–41. [PubMed] [Google Scholar]
- Regal J. F., Fraser D. G., Anderson D. E., Solem L. E. Enhancement of antigen-induced bronchoconstriction after intravascular complement activation with cobra venom factor. Reversal by granulocyte depletion. J Immunol. 1993 Apr 15;150(8 Pt 1):3496–3505. [PubMed] [Google Scholar]
- Regal J. F., Fraser D. G., Toth C. A. Role of the complement system in antigen-induced bronchoconstriction and changes in blood pressure in the guinea pig. J Pharmacol Exp Ther. 1993 Nov;267(2):979–988. [PubMed] [Google Scholar]
- Resnick M. B., Colgan S. P., Patapoff T. W., Mrsny R. J., Awtrey C. S., Delp-Archer C., Weller P. F., Madara J. L. Activated eosinophils evoke chloride secretion in model intestinal epithelia primarily via regulated release of 5'-AMP. J Immunol. 1993 Nov 15;151(10):5716–5723. [PubMed] [Google Scholar]
- Schudt C., Winder S., Forderkunz S., Hatzelmann A., Ullrich V. Influence of selective phosphodiesterase inhibitors on human neutrophil functions and levels of cAMP and Cai. Naunyn Schmiedebergs Arch Pharmacol. 1991 Dec;344(6):682–690. doi: 10.1007/BF00174752. [DOI] [PubMed] [Google Scholar]
- Schudt C., Winder S., Müller B., Ukena D. Zardaverine as a selective inhibitor of phosphodiesterase isozymes. Biochem Pharmacol. 1991 Jun 21;42(1):153–162. doi: 10.1016/0006-2952(91)90694-z. [DOI] [PubMed] [Google Scholar]
- Simasko S. M., Yan S. 3-Isobutyl-1-methylxanthine inhibits sustained calcium current independently of cyclic AMP in neuronal and endocrine cells. Mol Pharmacol. 1993 Sep;44(3):622–627. [PubMed] [Google Scholar]
- Souness J. E., Carter C. M., Diocee B. K., Hassall G. A., Wood L. J., Turner N. C. Characterization of guinea-pig eosinophil phosphodiesterase activity. Assessment of its involvement in regulating superoxide generation. Biochem Pharmacol. 1991 Jul 25;42(4):937–945. doi: 10.1016/0006-2952(91)90056-b. [DOI] [PubMed] [Google Scholar]
- Souness J. E., Maslen C., Scott L. C. Effects of solubilization and vanadate/glutathione complex on inhibitor potencies against eosinophil cyclic AMP-specific phosphodiesterase. FEBS Lett. 1992 May 11;302(2):181–184. doi: 10.1016/0014-5793(92)80435-j. [DOI] [PubMed] [Google Scholar]
- Sullivan P., Bekir S., Jaffar Z., Page C., Jeffery P., Costello J. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet. 1994 Apr 23;343(8904):1006–1008. doi: 10.1016/s0140-6736(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Terasaki W. L., Epstein P. M., Strada S. J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv Cyclic Nucleotide Res. 1979;10:69–92. [PubMed] [Google Scholar]
- Tomes C., Rossi S., Moreno S. Isobutylmethylxanthine and other classical cyclic nucleotide phosphodiesterase inhibitors affect cAMP-dependent protein kinase activity. Cell Signal. 1993 Sep;5(5):615–621. doi: 10.1016/0898-6568(93)90056-r. [DOI] [PubMed] [Google Scholar]
- Ukena D., Schudt C., Sybrecht G. W. Adenosine receptor-blocking xanthines as inhibitors of phosphodiesterase isozymes. Biochem Pharmacol. 1993 Feb 24;45(4):847–851. doi: 10.1016/0006-2952(93)90168-v. [DOI] [PubMed] [Google Scholar]
- Yukawa T., Ukena D., Kroegel C., Chanez P., Dent G., Chung K. F., Barnes P. J. Beta 2-adrenergic receptors on eosinophils. Binding and functional studies. Am Rev Respir Dis. 1990 Jun;141(6):1446–1452. doi: 10.1164/ajrccm/141.6.1446. [DOI] [PubMed] [Google Scholar]


