Abstract
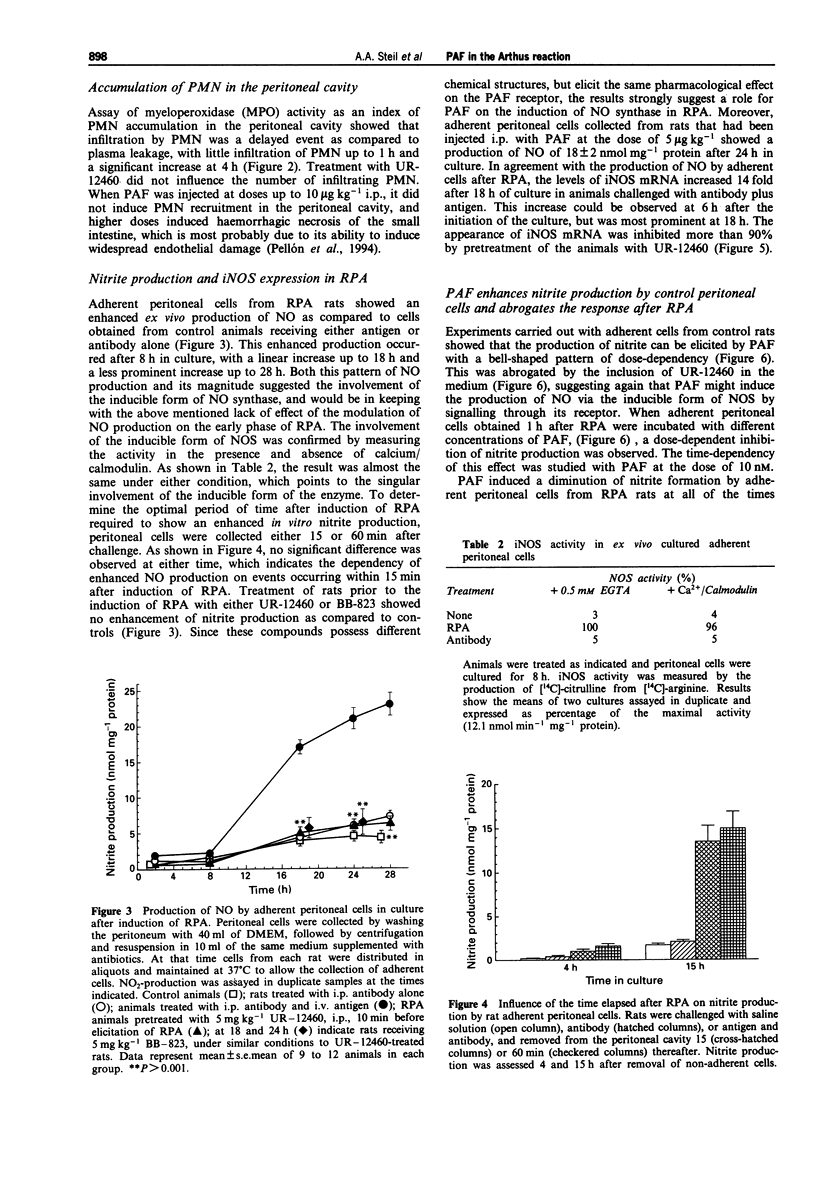
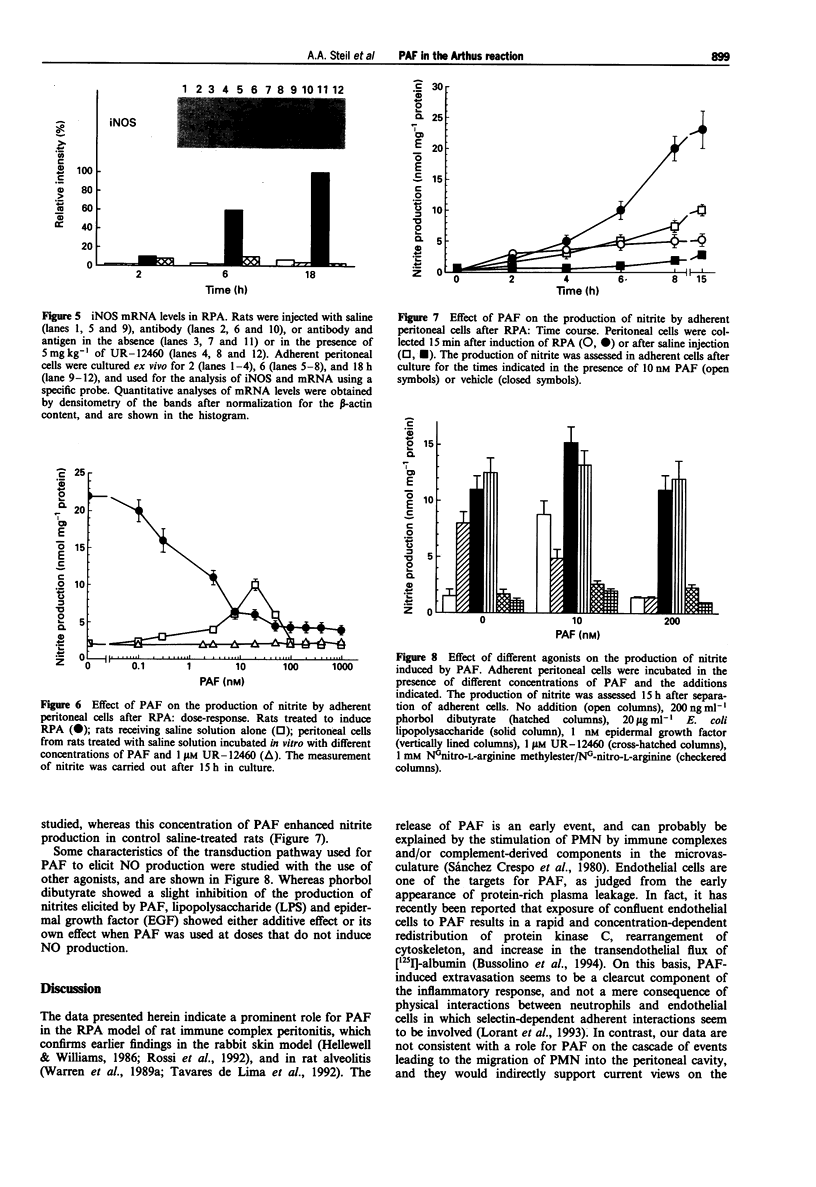
1. The involvement of platelet-activating factor (PAF) in immune complex-induced/polymorphonuclear-mediated tissue injury was studied by use of a reverse passive Arthus (RPA) model in the peritoneal cavity of rats. 2. Extravasation of protein-rich plasma, accumulation of polymorphonuclear leukocytes (PMN), and the production of nitric oxide (NO) by resident peritoneal mononuclear phagocytes were assayed. 3. Treatment of rats with either UR-12460 or BB-823, two compounds which possess different chemical structures, but elicit the same antagonistic effect on the PAF receptor, abrogated protein-rich plasma extravasation. In contrast, they did not show any effect on the accumulation of PMN. 4. Inhibition of NO production with both NG-mono methyl-L-arginine and NG-nitro-L-arginine failed to prevent protein-rich plasma extravasation. 5. The production of NO by peritoneal adherent cells following RPA was measured in cells maintained for 2 to 28 h in culture, and it was significantly increased in cells removed as early as 15 min after RPA induction, as compared to controls. 6. Addition of 10 nM PAF to the culture medium reduced the generation of NO by peritoneal cells from RPA rats, whereas this mediator enhanced NO production in cells from naive control animals. 7. Treatment with either UR-12460 or BB-823 prior to the induction of RPA produced an almost complete inhibition of NO production. 8. Assay of nitric oxide synthase activity in cell homogenates from peritoneal cells showed that the activity was due to the inducible form of the enzyme.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt H., Russell J. B., Kurose I., Kubes P., Granger D. N. Mediators of leukocyte adhesion in rat mesenteric venules elicited by inhibition of nitric oxide synthesis. Gastroenterology. 1993 Sep;105(3):675–680. doi: 10.1016/0016-5085(93)90882-d. [DOI] [PubMed] [Google Scholar]
- Bazan H. E., Tao Y., Bazan N. G. Platelet-activating factor induces collagenase expression in corneal epithelial cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8678–8682. doi: 10.1073/pnas.90.18.8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Deakin A. M., Follenfant R. L., Whittle B. J., Garland L. G. Role of oxygen radicals and arachidonic acid metabolites in the reverse passive Arthus reaction and carrageenin paw oedema in the rat. Br J Pharmacol. 1993 Oct;110(2):896–902. doi: 10.1111/j.1476-5381.1993.tb13897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., Silvagno F., Garbarino G., Costamagna C., Sanavio F., Arese M., Soldi R., Aglietta M., Pescarmona G., Camussi G. Human endothelial cells are targets for platelet-activating factor (PAF). Activation of alpha and beta protein kinase C isozymes in endothelial cells stimulated by PAF. J Biol Chem. 1994 Jan 28;269(4):2877–2886. [PubMed] [Google Scholar]
- Caplan M. S., Hedlund E., Hill N., MacKendrick W. The role of endogenous nitric oxide and platelet-activating factor in hypoxia-induced intestinal injury in rats. Gastroenterology. 1994 Feb;106(2):346–352. doi: 10.1016/0016-5085(94)90591-6. [DOI] [PubMed] [Google Scholar]
- Carceller E., Merlos M., Giral M., Almansa C., Bartrolí J., García-Rafanell J., Forn J. Synthesis and structure-activity relationships of 1-acyl-4-((2-methyl-3-pyridyl)cyanomethyl)piperazines as PAF antagonists. J Med Chem. 1993 Oct 1;36(20):2984–2997. doi: 10.1021/jm00072a019. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Nitric oxide: foe or friend to the injured brain? Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9741–9743. doi: 10.1073/pnas.90.21.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins P. D., Jose P. J., Williams T. J. The sequential generation of neutrophil chemoattractant proteins in acute inflammation in the rabbit in vivo. Relationship between C5a and proteins with the characteristics of IL-8/neutrophil-activating protein 1. J Immunol. 1991 Jan 15;146(2):677–684. [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hellewell P. G., Williams T. J. A specific antagonist of platelet-activating factor suppresses oedema formation in an Arthus reaction but not oedema induced by leukocyte chemoattractants in rabbit skin. J Immunol. 1986 Jul 1;137(1):302–307. [PubMed] [Google Scholar]
- Henson P. M., Zanolari B., Schwartzman N. A., Hong S. R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978 Sep;121(3):851–855. [PubMed] [Google Scholar]
- Hiki K., Yui Y., Hattori R., Eizawa H., Kosuga K., Kawai C. Three regulation mechanisms of nitric oxide synthase. Eur J Pharmacol. 1991 Feb 25;206(2):163–164. doi: 10.1016/0922-4106(91)90026-e. [DOI] [PubMed] [Google Scholar]
- Hortelano S., Genaro A. M., Boscá L. Phorbol esters induce nitric oxide synthase activity in rat hepatocytes. Antagonism with the induction elicited by lipopolysaccharide. J Biol Chem. 1992 Dec 15;267(35):24937–24940. [PubMed] [Google Scholar]
- Ierino F. L., Powell M. S., McKenzie I. F., Hogarth P. M. Recombinant soluble human Fc gamma RII: production, characterization, and inhibition of the Arthus reaction. J Exp Med. 1993 Nov 1;178(5):1617–1628. doi: 10.1084/jem.178.5.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Nathan C. F. Nitrite production by stimulated human polymorphonuclear leukocytes supplemented with azide and catalase. Biochem Biophys Res Commun. 1993 Nov 30;197(1):192–196. doi: 10.1006/bbrc.1993.2459. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Reuveny E., Slesinger P. A., Jan Y. N., Jan L. Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993 Aug 26;364(6440):802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- Kunz D., Mühl H., Walker G., Pfeilschifter J. Two distinct signaling pathways trigger the expression of inducible nitric oxide synthase in rat renal mesangial cells. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5387–5391. doi: 10.1073/pnas.91.12.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorant D. E., Topham M. K., Whatley R. E., McEver R. P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Inflammatory roles of P-selectin. J Clin Invest. 1993 Aug;92(2):559–570. doi: 10.1172/JCI116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazer B., Domenico J., Sawami H., Gelfand E. W. Platelet-activating factor induces an increase in intracellular calcium and expression of regulatory genes in human B lymphoblastoid cells. J Immunol. 1991 Mar 15;146(6):1914–1920. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Moncada S., Ward P. A. Protective effects of inhibitors of nitric oxide synthase in immune complex-induced vasculitis. Br J Pharmacol. 1992 Dec;107(4):1159–1162. doi: 10.1111/j.1476-5381.1992.tb13423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Watson S. R., Fennie C., Ward P. A. Protective effects of selectin chimeras in neutrophil-mediated lung injury. J Immunol. 1993 Dec 1;151(11):6410–6417. [PubMed] [Google Scholar]
- Mühl H., Kunz D., Pfeilschifter J. Expression of nitric oxide synthase in rat glomerular mesangial cells mediated by cyclic AMP. Br J Pharmacol. 1994 May;112(1):1–8. doi: 10.1111/j.1476-5381.1994.tb13019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellón M. I., Steil A. A., Furió V., Sánchez Crespo M. Study of the effector mechanism involved in the production of haemorrhagic necrosis of the small intestine in rat passive anaphylaxis. Br J Pharmacol. 1994 Aug;112(4):1101–1108. doi: 10.1111/j.1476-5381.1994.tb13197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peunova N., Enikolopov G. Amplification of calcium-induced gene transcription by nitric oxide in neuronal cells. Nature. 1993 Jul 29;364(6436):450–453. doi: 10.1038/364450a0. [DOI] [PubMed] [Google Scholar]
- Pons F., Williams T. J., Warren J. B. Nitric oxide, but not interleukin-1, mediates the local blood flow response to lipopolysaccharide in rabbit skin. Eur J Pharmacol. 1993 Aug 3;239(1-3):23–30. doi: 10.1016/0014-2999(93)90971-j. [DOI] [PubMed] [Google Scholar]
- Rossi A. G., Norman K. E., Donigi-Gale D., Shoupe T. S., Edwards R., Williams T. J. The role of complement, platelet-activating factor and leukotriene B4 in a reversed passive Arthus reaction. Br J Pharmacol. 1992 Sep;107(1):44–49. doi: 10.1111/j.1476-5381.1992.tb14461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattner M. A., Geffner J. R., Isturiz M. A., Lazzari M. A. Inhibition of human platelet activation by polymorphonuclear leukocytes. Br J Pharmacol. 1990 Oct;101(2):253–256. doi: 10.1111/j.1476-5381.1990.tb12696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Warner T. D., Nakane M., Förstermann U., Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol. 1992 Apr;41(4):615–624. [PubMed] [Google Scholar]
- Squinto S. P., Block A. L., Braquet P., Bazan N. G. Platelet-activating factor stimulates a fos/jun/AP-1 transcriptional signaling system in human neuroblastoma cells. J Neurosci Res. 1989 Dec;24(4):558–566. doi: 10.1002/jnr.490240414. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C., Wu C. C., Mitchell J. A., Gross S. S., Thiemermann C., Vane J. R. Platelet-activating factor contributes to the induction of nitric oxide synthase by bacterial lipopolysaccharide. Circ Res. 1993 Dec;73(6):991–999. doi: 10.1161/01.res.73.6.991. [DOI] [PubMed] [Google Scholar]
- Sánchez-Crespo M., Alonso F., Egido J. Platelet-activating factor in anaphylaxis and phagocytosis. I. Release from human peripheral polymorphonuclears and monocytes during the stimulation by ionophore A23187 and phagocytosis but not from degranulating basophils. Immunology. 1980 Aug;40(4):645–655. [PMC free article] [PubMed] [Google Scholar]
- Warren J. S., Mandel D. M., Johnson K. J., Ward P. A. Evidence for the role of platelet-activating factor in immune complex vasculitis in the rat. J Clin Invest. 1989 Feb;83(2):669–678. doi: 10.1172/JCI113931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. S. Relationship between interleukin-1 beta and platelet-activating factor in the pathogenesis of acute immune complex alveolitis in the rat. Am J Pathol. 1992 Sep;141(3):551–560. [PMC free article] [PubMed] [Google Scholar]
- Warren J. S., Yabroff K. R., Remick D. G., Kunkel S. L., Chensue S. W., Kunkel R. G., Johnson K. J., Ward P. A. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J Clin Invest. 1989 Dec;84(6):1873–1882. doi: 10.1172/JCI114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Wink D. A., Hanbauer I., Krishna M. C., DeGraff W., Gamson J., Mitchell J. B. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Yui Y., Hattori R., Kosuga K., Eizawa H., Hiki K., Ohkawa S., Ohnishi K., Terao S., Kawai C. Calmodulin-independent nitric oxide synthase from rat polymorphonuclear neutrophils. J Biol Chem. 1991 Feb 25;266(6):3369–3371. [PubMed] [Google Scholar]
- Zhang Y., Ramos B. F., Jakschik B. A. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992 Dec 18;258(5090):1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- de Lima W. T., Sirois P., Jancar S. Immune-complex alveolitis in the rat: evidence for platelet activating factor and leukotrienes as mediators of the vascular lesions. Eur J Pharmacol. 1992 Mar 17;213(1):63–70. doi: 10.1016/0014-2999(92)90233-t. [DOI] [PubMed] [Google Scholar]



