Abstract
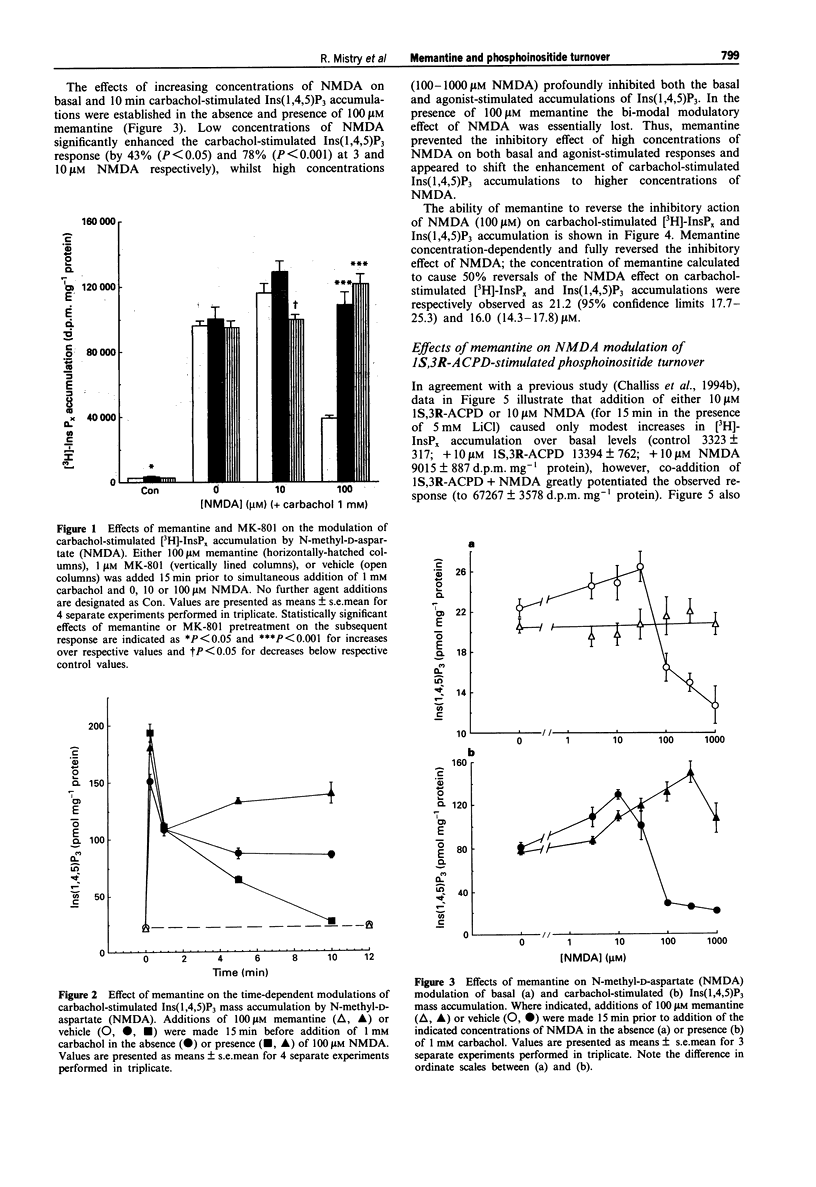
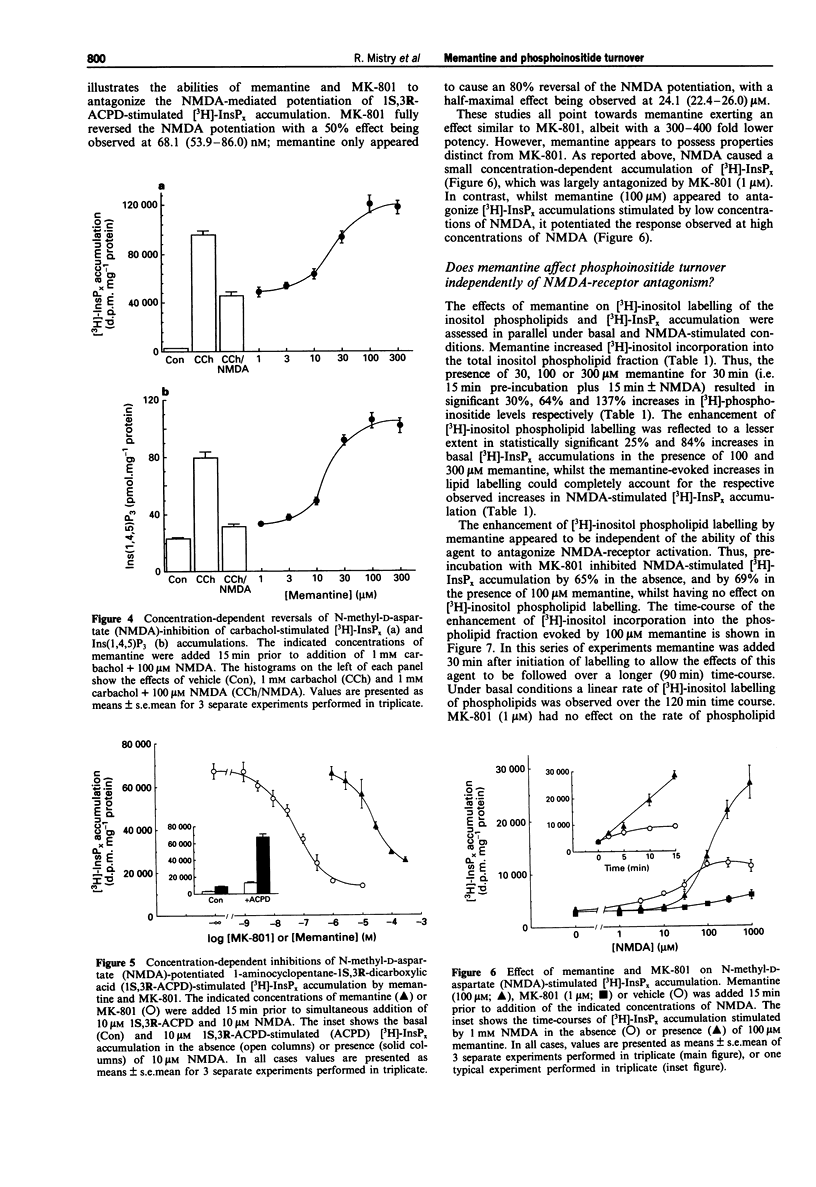
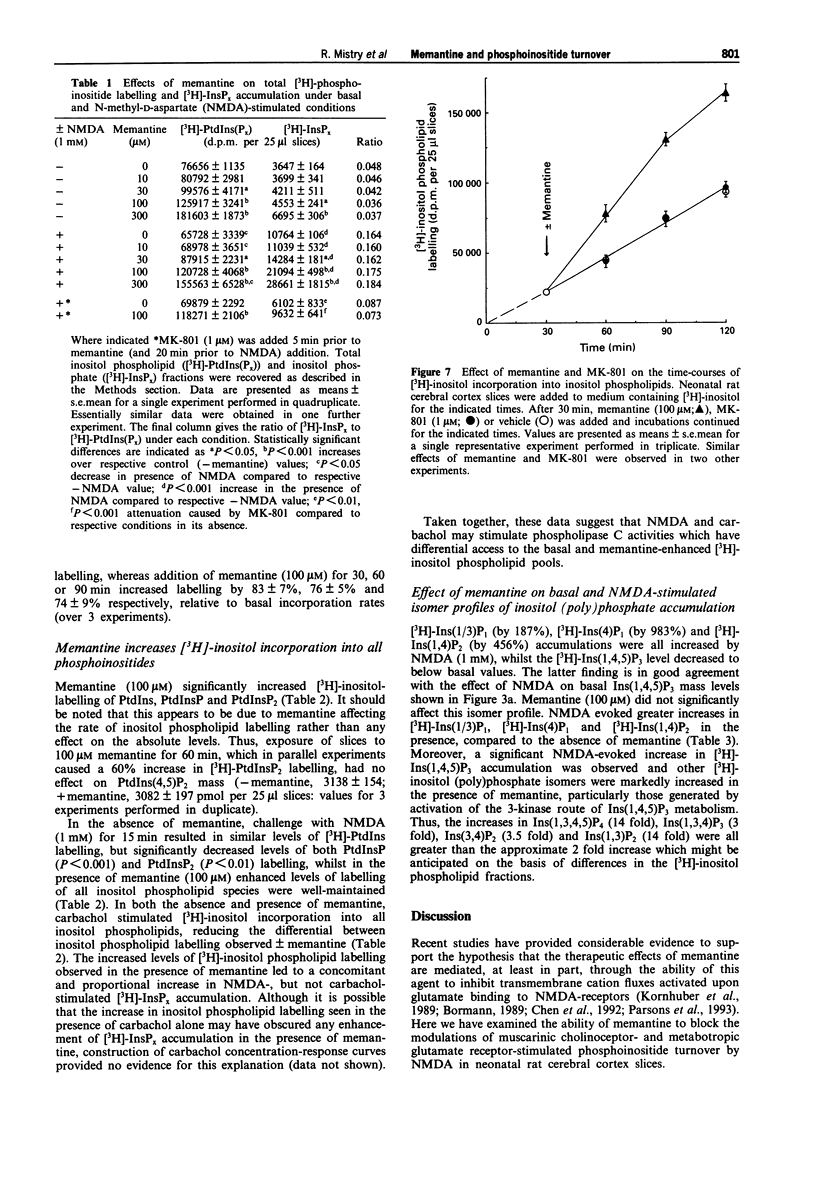
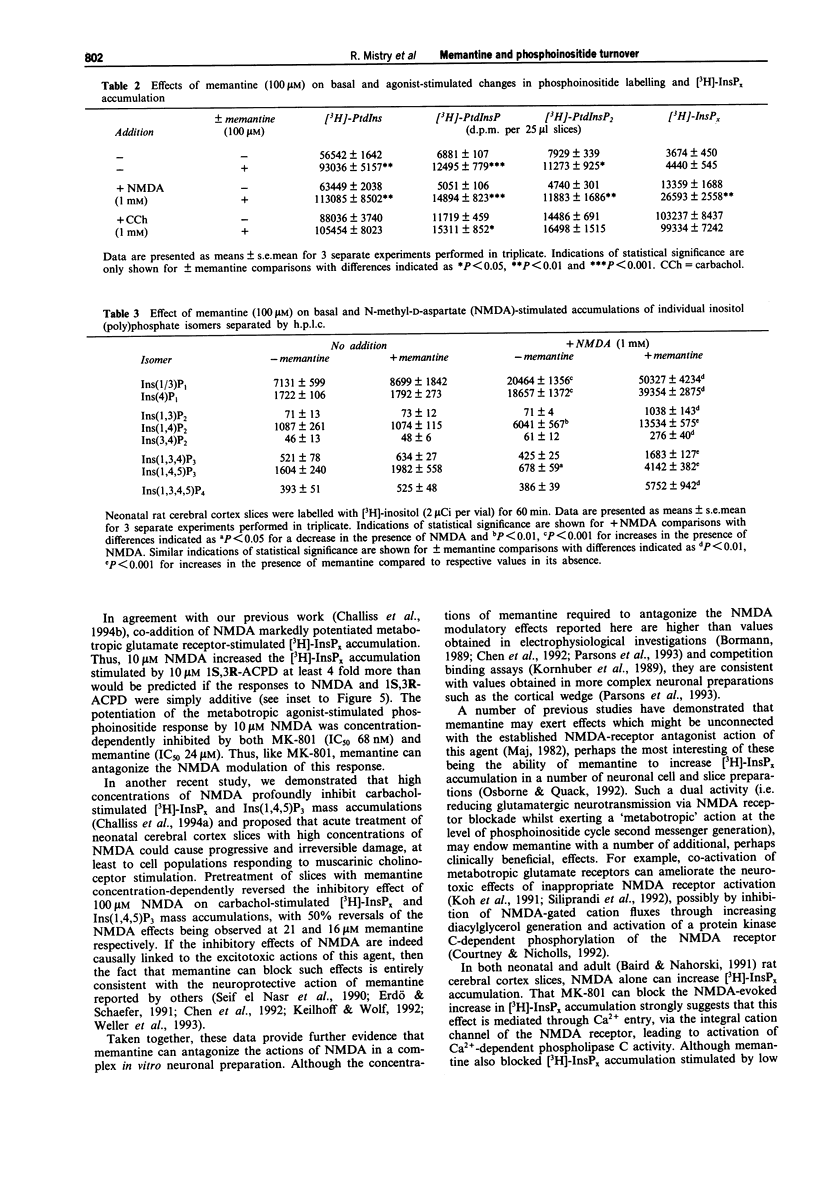
1. The ability of memantine (1-amino-3,5-dimethyladamantane) to antagonize the modulatory effects of N-methyl-D-aspartate (NMDA) on phosphoinositide turnover stimulated by muscarinic cholinoceptor- and metabotropic glutamate receptor-agonists has been examined in neonatal rat cerebral cortex slices. 2. Memantine antagonized the inhibitory effect of NMDA (100 microM) on both total [3H]-inositol phosphate ([3H]-InsPx) and inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) mass accumulations stimulated by carbachol (1 mM) with EC50 values of 21 and 16 microM respectively. 3. Memantine concentration-dependently antagonized (IC50 24 microM) the ability of NMDA (10 microM) to potentiate [3H]-InsPx accumulation in response to a sub-maximal concentration of the metabotropic glutamate receptor agonist, 1S,3R-ACPD (10 microM). 4. The small (approx. 3 fold), concentration-dependent increase in [3H]-InsPx accumulation stimulated by NMDA was completely antagonized by the prototypic NDMA receptor-channel blocker, MK-801 (1 microM) at all concentrations of NDMA studied (1-1000 microM). In contrast, antagonism by memantine (100 microM) was observed only at low concentrations of NMDA (1-10 microM), whilst [3H]-InsPx accumulation stimulated by high concentrations of NMDA (300-1000 microM) was markedly enhanced by memantine. 5. Assessment of the incorporation of [3H]-inositol into inositol phospholipids revealed that memantine (100 microM) caused an approximate 2 fold increase in the labelling of phosphatidylinositol, phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird J. G., Nahorski S. R. Stimulatory and inhibitory effects of N-methyl-D-aspartate on 3H-inositol polyphosphate accumulation in rat cortical slices. J Neurochem. 1991 Aug;57(2):629–635. doi: 10.1111/j.1471-4159.1991.tb03794.x. [DOI] [PubMed] [Google Scholar]
- Batty I. H., Letcher A. J., Nahorski S. R. Accumulation of inositol polyphosphate isomers in agonist-stimulated cerebral-cortex slices. Comparison with metabolic profiles in cell-free preparations. Biochem J. 1989 Feb 15;258(1):23–32. doi: 10.1042/bj2580023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J. Memantine is a potent blocker of N-methyl-D-aspartate (NMDA) receptor channels. Eur J Pharmacol. 1989 Aug 3;166(3):591–592. doi: 10.1016/0014-2999(89)90385-3. [DOI] [PubMed] [Google Scholar]
- Challis R. A., Mistry R., Gray D. W., Nahorski S. R. Modulation of muscarinic cholinoceptor-stimulated inositol 1,4,5-trisphosphate accumulation by N-methyl-D-aspartate in neonatal rat cerebral cortex. Neuropharmacology. 1994 Jan;33(1):15–25. doi: 10.1016/0028-3908(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Batty I. H., Nahorski S. R. Mass measurements of inositol(1,4,5)trisphosphate in rat cerebral cortex slices using a radioreceptor assay: effects of neurotransmitters and depolarization. Biochem Biophys Res Commun. 1988 Dec 15;157(2):684–691. doi: 10.1016/s0006-291x(88)80304-8. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Mistry R., Gray D. W., Nahorski S. R. Modulatory effects of NMDA on phosphoinositide responses evoked by the metabotropic glutamate receptor agonist 1S,3R-ACPD in neonatal rat cerebral cortex. Br J Pharmacol. 1994 May;112(1):231–239. doi: 10.1111/j.1476-5381.1994.tb13057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. S., Pellegrini J. W., Aggarwal S. K., Lei S. Z., Warach S., Jensen F. E., Lipton S. A. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992 Nov;12(11):4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M. J., Nicholls D. G. Interactions between phospholipase C-coupled and N-methyl-D-aspartate receptors in cultured cerebellar granule cells: protein kinase C mediated inhibition of N-methyl-D-aspartate responses. J Neurochem. 1992 Sep;59(3):983–992. doi: 10.1111/j.1471-4159.1992.tb08339.x. [DOI] [PubMed] [Google Scholar]
- Ditzler K. Efficacy and tolerability of memantine in patients with dementia syndrome. A double-blind, placebo controlled trial. Arzneimittelforschung. 1991 Aug;41(8):773–780. [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdö S. L., Schäfer M. Memantine is highly potent in protecting cortical cultures against excitotoxic cell death evoked by glutamate and N-methyl-D-aspartate. Eur J Pharmacol. 1991 Jun 6;198(2-3):215–217. doi: 10.1016/0014-2999(91)90625-z. [DOI] [PubMed] [Google Scholar]
- Grossmann W., Schütz W. Memantin und neurogene Blasenstörungen im Rahmen spastischer Zustandsbilder. Arzneimittelforschung. 1982;32(10):1273–1276. [PubMed] [Google Scholar]
- Görtelmeyer R., Erbler H. Memantine in the treatment of mild to moderate dementia syndrome. A double-blind placebo-controlled study. Arzneimittelforschung. 1992 Jul;42(7):904–913. [PubMed] [Google Scholar]
- Keilhoff G., Wolf G. Memantine prevents quinolinic acid-induced hippocampal damage. Eur J Pharmacol. 1992 Sep 4;219(3):451–454. doi: 10.1016/0014-2999(92)90487-o. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Palmer E., Cotman C. W. Activation of the metabotropic glutamate receptor attenuates N-methyl-D-aspartate neurotoxicity in cortical cultures. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9431–9435. doi: 10.1073/pnas.88.21.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J., Bormann J., Retz W., Hübers M., Riederer P. Memantine displaces [3H]MK-801 at therapeutic concentrations in postmortem human frontal cortex. Eur J Pharmacol. 1989 Aug 3;166(3):589–590. doi: 10.1016/0014-2999(89)90384-1. [DOI] [PubMed] [Google Scholar]
- Lupp A., Lücking C. H., Koch R., Jackisch R., Feuerstein T. J. Inhibitory effects of the antiparkinsonian drugs memantine and amantadine on N-methyl-D-aspartate-evoked acetylcholine release in the rabbit caudate nucleus in vitro. J Pharmacol Exp Ther. 1992 Nov;263(2):717–724. [PubMed] [Google Scholar]
- Maj J. Die Wirkung von Memantin auf zentrale Neurotransmittersysteme. Eine Zusammenfassung der Ergebnisse. Arzneimittelforschung. 1982;32(10):1256–1259. [PubMed] [Google Scholar]
- Maj J., Sowińska H., Baran L., Sarnek J. Pharmacological effects of 1,3-dimethyl-5-aminoadamantane, a new adamantane derivative. Eur J Pharmacol. 1974 Apr;26(1):9–14. doi: 10.1016/0014-2999(74)90067-3. [DOI] [PubMed] [Google Scholar]
- Monaco M. E., Adelson J. R. Evidence for coupling of resynthesis to hydrolysis in the phosphoinositide cycle. Biochem J. 1991 Oct 15;279(Pt 2):337–341. doi: 10.1042/bj2790337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne N. N., Quack G. Memantine stimulates inositol phosphates production in neurones and nullifies N-methyl-D-aspartate-induced destruction of retinal neurones. Neurochem Int. 1992 Oct;21(3):329–336. doi: 10.1016/0197-0186(92)90183-r. [DOI] [PubMed] [Google Scholar]
- Parsons C. G., Gruner R., Rozental J., Millar J., Lodge D. Patch clamp studies on the kinetics and selectivity of N-methyl-D-aspartate receptor antagonism by memantine (1-amino-3,5-dimethyladamantan). Neuropharmacology. 1993 Dec;32(12):1337–1350. doi: 10.1016/0028-3908(93)90029-3. [DOI] [PubMed] [Google Scholar]
- Schneider E., Fischer P. A., Clemens R., Balzereit F., Fünfgeld E. W., Haase H. J. Wirkungen oraler Memantin-Gaben auf die Parkinson-Symptomatik. Ergebnisse einer placebo-kontrollierten Multicenter-Studie. Dtsch Med Wochenschr. 1984 Jun 22;109(25):987–990. doi: 10.1055/s-2008-1069311. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D. Manganese stimulates the incorporation of [3H]inositol into a pool of phosphatidylinositol in brain that is not coupled to agonist-induced hydrolysis. J Neurochem. 1985 Nov;45(5):1481–1486. doi: 10.1111/j.1471-4159.1985.tb07216.x. [DOI] [PubMed] [Google Scholar]
- Seif el Nasr M., Peruche B., Rossberg C., Mennel H. D., Krieglstein J. Neuroprotective effect of memantine demonstrated in vivo and in vitro. Eur J Pharmacol. 1990 Aug 21;185(1):19–24. doi: 10.1016/0014-2999(90)90206-l. [DOI] [PubMed] [Google Scholar]
- Shears S. B. Regulation of the metabolism of 1,2-diacylglycerols and inositol phosphates that respond to receptor activation. Pharmacol Ther. 1991;49(1-2):79–104. doi: 10.1016/0163-7258(91)90023-f. [DOI] [PubMed] [Google Scholar]
- Siliprandi R., Lipartiti M., Fadda E., Sautter J., Manev H. Activation of the glutamate metabotropic receptor protects retina against N-methyl-D-aspartate toxicity. Eur J Pharmacol. 1992 Aug 14;219(1):173–174. doi: 10.1016/0014-2999(92)90598-x. [DOI] [PubMed] [Google Scholar]
- Thomas G. M., Cunningham E., Fensome A., Ball A., Totty N. F., Truong O., Hsuan J. J., Cockcroft S. An essential role for phosphatidylinositol transfer protein in phospholipase C-mediated inositol lipid signaling. Cell. 1993 Sep 10;74(5):919–928. doi: 10.1016/0092-8674(93)90471-2. [DOI] [PubMed] [Google Scholar]
- Weller M., Finiels-Marlier F., Paul S. M. NMDA receptor-mediated glutamate toxicity of cultured cerebellar, cortical and mesencephalic neurons: neuroprotective properties of amantadine and memantine. Brain Res. 1993 Jun 4;613(1):143–148. doi: 10.1016/0006-8993(93)90464-x. [DOI] [PubMed] [Google Scholar]
- Wesemann W., Sontag K. H., Maj J. Zur Pharmakodynamik und Pharmakokinetik des Memantin. Arzneimittelforschung. 1983;33(8):1122–1134. [PubMed] [Google Scholar]
- Yandrasitz J. R., Segal S. The effect of MnCl2 on the basal and acetylcholine-stimulated turnover of phosphatidylinositol in synaptosomes. FEBS Lett. 1979 Dec 1;108(1):279–282. doi: 10.1016/0014-5793(79)81228-4. [DOI] [PubMed] [Google Scholar]


