In response to changes in environmental conditions, animals and humans alike can initiate physiological regulatory mechanisms to assure survival. For example, when food is scarce, the body becomes “thrifty”, and conversely, when it is abundant, physiological “unthrifty” mechanisms are initiated. Recent work has uncovered novel cellular mechanisms of fuel sensing that contribute to these shifts and has established a potential relationship between these responses and the common metabolic phenotype of insulin resistance.
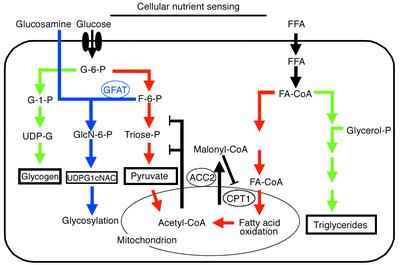
The first such mechanism to be identified depends on malonyl Co-A, which allosterically inhibits carnitine palmitoyl transferase (CPT1). Because this enzyme controls mitochondrial uptake, and hence oxidation, of long-chain fatty acyl-CoAs (see Ruderman for review, ref. 1), malonyl-CoA can act as a fuel sensor to regulate the rate of fatty acid oxidation in muscle (Figure 1). Ten years ago, Marshall et al. (2) suggested that another fuel-sensing mechanism, this one dependent on the hexosamine biosynthesis pathway (blue arrows in Figure 1), mediates the cellular response to excess environmental glucose. These authors proposed that metabolism of glucose by this route, which typically represents 1-3% of total glucose metabolism, generates a cellular satiety signal that leads to decreased glucose uptake by insulin-sensitive cells.
Figure 1.
Two cellular fuel-sensing pathways. The hexosamine biosynthesis pathway and the malonyl Co-A system are proposed to modulate energy consumption by muscle and other tissues in response to changing levels of metabolic fuels. Following its entry into the cell via the glucose transport system, glucose is phosphorylated into glucose-6-phosphate (G-6-P), which is primarily utilized by the pathways of glycogen synthesis and glycolysis. Only 1–3% of the glucose entering the cells is diverted to glucosamine-6-phostate by the rate-limiting enzyme glutamine:fructose-6-phosphate amidotransferase (GFAT) (blue arrows). The end product of this pathway, uridinediphosphoglucose-N-acetylglucosamine (UDP-GlcNAc) serves as substrate for virtually all glycosylation pathways in the cells. Glucosamine enters the pathway directly after the rate-limiting step of GFAT, therefore mimicking a signal of excess glucose or nutrient. The second fuel-sensing pathway is that of malonyl-CoA. Increased cytosolic citrate from both the Krebs cycle and beta-oxidation contribute to increased Acetyl-CoA. Acetyl-CoA is the precursor of malonyl-CoA, a conversion that involves acetyl-CoA carboxylase 2 (ACC2). The primary role of malonyl-CoA is to regulate the rate of fat oxidation via its allosteric inhibition of carnitine palmitoyl transferase (CPT1), the enzyme responsible for converting long-chain fatty acyl-CoA into long-chain acyl-carnitine. Because the availability of Acetyl-CoA and the activity of ACC2 can each influence the concentration of malonyl-CoA, both can alter the balance between fat storage and fat oxidation.
In this issue of the JCI, Obici et al. (3) propose that the hexosamine pathway is not only involved in glucose metabolism and insulin resistance, but may also be one of the biochemical links between nutrient availability and cellular energy metabolism. The activation or inhibition of this fuel sensing pathway may, therefore, play an important role in the regulation of energy balance. However, their present data raise significant questions about the role of transcriptional regulation in the adaptive response to energy surfeit.
Energy balance and the “thrifty genotype”
Historically, most human populations have evolved in environments in which large amounts of physical effort were required to obtain food in limited supply. Thus, it is not surprising that the human genome harbors many genes that predispose to positive energy balance and obesity and fewer that protect against the effect of affluence. The tendency of populations to gain weight under current conditions of plenty should therefore be considered as a normal physiologic response to what has been called an “obesigenic” environment. Large differences in individuals’ propensity to weight gain persist in the face of this general trend (4, 5), probably because the “thrifty genotype”, as James Neel termed it, is distributed unevenly among individuals (6, 7).
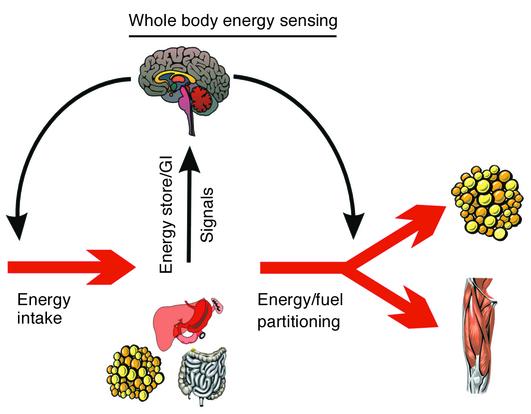
One hundred years ago, Neumann made the seminal observation that the increase in body weight in response to overfeeding is not proportional to the excess ingested energy (8). To explain this discrepancy, he hypothesized that some of the energy ingested, in excess of normal requirements, was dissipated as heat or “Luxuskonsumption”. This was the first description of what is now referred to as adaptive thermogenesis. Over the past five decades, scientists have tried to identify the mechanisms underlying the variability in adaptive thermogenesis and its consequence on weight gain in response to overfeeding. First, at the whole-body level, it became clear that energy surfeit can be sensed and that signals are sent to central controllers. These controllers then orchestrate a physiological response, which tends to limit food intake and increase energy metabolism (Figure 2). This feedback model, originally proposed by Bray (9), still represents the working hypothesis that is used to explain the large variability in body weight gain in response to excess energy intake. According to this model, in response to overfeeding, the gastro-intestinal tract and the adipose tissue, among others, provide endocrine and neural signals to brain controllers of energy balance. In response, these controllers engage a series of efferent neural and neuroendocrine signals that are specifically geared to counteract the excess caloric intake. These signals eventually decrease appetite and/or increase energy expenditure, thus causing a decrease in the energy stored in the body. Conversely, during periods of food restriction, opposing signals protect the body against energy loss by increasing hunger and food-seeking behaviors and decreasing energy expenditure. Any component of this feedback loop can influence the robustness of the adaptive response. More recent research has focused on the potential molecular mechanisms that explain this response to fuel availability at the cellular level.
Figure 2.
The negative feedback model for the regulation of body weight. In this model, peripheral signals from energy stores (adipose tissue, muscle, and liver) as well as hormonal and gastrointestinal signals act on the central controllers in the brain, indicating the state of the external and internal environment as they relate to food, metabolic rate, and activity behaviors. In turn, the central controllers integrate these signals and transduce these messages into efferent signals governing the behavioral search for the acquisition of food, as well as modulating its subsequent deposition into energy storage compartments, such as adipose tissue, liver, and muscle. Afferent signals, efferent signals, and central controllers can all be influenced by an organism’s genetic makeup, hence the inter-individual variability in weight change in response to a perturbed energy balance.
Glucose sensing mechanisms
Because the availability of carbon sources can fluctuate widely, organisms from prokaryotes to humans require means to sense the level of glucose, the major source of energy in most cases. For example, two different glucose-sensing signal transduction pathways have been identified in the yeast Saccharomyces cerevisiae, one for repression and one for induction of gene expression (10). Mammals have developed an indirect mechanism by which the beta cells in the pancreas modulate insulin secretion by monitoring glucokinase and ATP changes, which in turn reflect the amount of intracellular glucose that undergoes glycolysis (11). Other mammalian cell types can also sense glucose and respond by repressing or inducing the necessary genes for adaptive responses.
The 10-year-old hypothesis that cells use hexosamine flux as a glucose- and satiety-sensing pathway has gained momentum (2). In skeletal muscle and adipose tissue, following glucose transport and phosphorylation, glucose-6-phosphate is primarily used for glycogenesis or glycolysis (Figure 1). A small proportion, however, is shunted to the hexosamine pathway by the rate-limiting enzyme glutamine:fructose-6-phosphate amidotransferase (GFAT), which converts fructose-6-phosphate to glucosamine-6-phosphate. Because the end product of this pathway, uridinediphosphoglucose-N-acetylglucosamine (UDP-GlcNAc), serves as a substrate for most glycosylation pathways, the flux through the hexosamine pathway could well affect the stability and activity of many proteins, ultimately altering cellular patterns of gene expression.
As reviewed by Rossetti (12), several studies have shown that increased flux of fructose-6-phosphate into the hexosamine biosynthesis pathway decreases glucose uptake in vivo in animals and in vitro in isolated cells. Similarly, when GFAT is over-expressed in transgenic animals, the skeletal muscle becomes insulin-resistant, β cells secrete excess insulin, and the liver synthesizes excess fatty acids in parallel to the increased flux through the hexosamine pathway (13). Consistent with the role of the hexosamine biosynthesis pathway as a fuel-sensing system, glucosamine infusion during a hyperinsulinemic euglycemic clamp, which bypasses the GFAT-dependent step (Figure 1), causes a marked decrease in glucose uptake (14). Whether increased availability of free fatty acids causes insulin resistance via an increased flux into the hexosamine pathway is still controversial (15, 16). The effect of glucosamine infusion on insulin sensitivity in humans is also unclear; one study found a modest decrease in insulin sensitivity (17), but another did not (18).
Gene expression and cellular energy expenditure
The new study by Obici et al. (3) emphasizes that the hexosamine biosynthesis pathway is not only a major mediator of carbohydrate metabolism, but may also represent a biochemical link between nutrient availability and the molecular cellular response in terms of energy expenditure. The authors tested the hypothesis that the hexosamine biosynthesis pathway represents a signal of energy “overflow,” which, they proposed, would initiate compensatory responses in skeletal muscle gene expression. Employing their previously developed paradigm of hyperinsulinemic euglycemic clamp in association with glucosamine infusion to mimic nutrient excess, they first measured muscle gene expression using microarrays. After a preliminary exploration of genes that were up- or down-regulated, they confirmed their findings by Northern blots. Surprisingly, they did not observe an increase, but rather a decrease in the expression of mRNAs encoding crucial mitochondrial proteins involved in oxidative phosphorylation and substrate oxidation. In particular, glucosamine down-regulated expression of one or more subunits in all but one of the respiratory chain complexes and of various gene products involved in fatty acid oxidation, mitochondrial substrate shuttling, or the Krebs cycle. On the other hand, the expression of the same genes (plus uncoupling protein-1) was increased in brown adipose tissue.
Consistent with the down-regulation of energy expenditure genes in muscle following glucosamine infusion, the rats exhibited a decrease in oxygen consumption and energy expenditure in the hours following the clamp procedure. To test this association in a more physiological manner, Obici et al. tested the effects of overfeeding in vivo. Although they found no evidence that the defined set of genes were upregulated in brown adipose tissue under these conditions, they did indeed confirm that they were down regulated in skeletal muscle. Counter to the glucosamine infusion paradigm, however, oxygen consumption was not decreased (on the contrary, as expected it was increased by 7%) in response to overfeeding. The regulation of the previously identified set of mRNAs thus appears not to be part of the physiological process of increased energy dissipation, at least not in muscle cells under conditions of overfeeding. This unexpected result may suggest that the acute response to overfeeding or glucosamine excess is controlled post-transcriptionally. Conceivably, the transcriptional response they observe serves another purpose in energy homeostasis, perhaps setting the stage for the muscle cells to return to normal or even lower levels of energy expenditure once the surfeit has passed.
Outstanding questions
As with any new discovery, the study by Rossetti’s laboratory provides more questions than answers. The authors conclude that “in keeping with the Neel’s thrifty genotype hypothesis, a sustained increase in the availability of nutrients may trigger biological actions designed to increase the efficiency of energy storage by limiting fuel oxidation and ATP production”. However, a thrifty organism, as envisioned by Neel, should respond by a decline in energy metabolism during times of famine, and an increase in energy metabolism (even though modest) during times of plenty (19). Clearly, the clamp/glucosamine paradigm did not cause the expected increase in energy expenditure during a condition mimicking energy excess.
Why should the expression of genes involved in energy dissipation decrease in a situation known to increase energy expenditure? It should be noted that the authors did not measure energy metabolism during the procedure but only during the 17 hours following the clamp, so the effects of the gene down-regulation they observed may not correlate with the expected physiological response. Interestingly, in the more physiological situation of overfeeding, the adaptive response to energy excess was an increase and not a decrease in energy expenditure. Oxygen consumption increased by 7% in response to overfeeding, in parallel to a moderate 35% increase in the muscle level of UDP-GlcNAc, the proposed cellular signal of energy surfeit. It is therefore conceivable that the duration and magnitude of the overfeeding was not sufficient to cause a larger stimulation of the hexosamine pathway, as evidenced by the lack of stimulation of UCP1 and the modest increase in energy expenditure. The discrepancy between the responses in skeletal muscle versus brown adipose tissue is intriguing and implies that gene regulation in response to a change in the hexosamine pathway flux is tissue-specific. However, it is likely that both tissues participate in concert in the adaptive response to overfeeding.
This report raises many questions regarding the importance of the hexosamine fuel-sensing pathway in the variability among strains of animals or among individuals in their response to feast or famine. Is this pathway induced differently in obesity-prone and obesity-resistant animals? Does the composition of the diet influence the intracellular concentration of the endproduct of the pathway (UDP-GlcNAc) in a way paralleling the changes in metabolic gene expression? Is the response to overfeeding influenced by the state of insulin resistance of the animals? Perhaps so, since diabetic animals presumably have high intracellular concentrations of UDP-GlcNAc. Is the hexosamine pathway equally important in other important tissue for energy balance, such as the liver? Since changes in gene expression are tissue-specific, studies are necessary to fully understand the molecular mechanisms by which the hexosamine biosynthesis pathway modulates the expression of genes involved in energy metabolism. The hexosamine biosynthesis pathway has also been shown to regulate leptin expression and secretion in adipose and muscle cells alike (20). Is there an indirect role of this pathway on body weight regulation via regulation of leptin biosynthesis? All these questions will need clarification. The answers will help narrow the gap in our understanding of whole-body physiology and of molecular mechanisms that control energy storage and consumption in muscles and other key tissues.
Footnotes
See the related article beginning on page 1599.
References
- 1.Ruderman NB, et al. Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol. 1999;276:E1–E18. doi: 10.1152/ajpendo.1999.276.1.E1. [DOI] [PubMed] [Google Scholar]
- 2.Marshall S, Bacote V, Traxinger RR. Complete inhibition of glucose-induced desensitization of the glucose transport system by inhibitors of mRNA synthesis. Evidence for rapid turnover of glutamine:fructose-6-phosphate amidotransferase. J Biol Chem. 1991;266:10155–10161. [PubMed] [Google Scholar]
- 3.Obici S, et al. Identification of a biochemical link between energy intake and energy expenditure. J Clin Invest. 2002;109:1599–1605. doi:10.1172/JCI200215258. doi: 10.1172/JCI15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchard C, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 5.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 6.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–363. [PMC free article] [PubMed] [Google Scholar]
- 7.Neel JV. The “thrifty genotype” in 1998. Nutr Rev. 1999;57:S2–S9. doi: 10.1111/j.1753-4887.1999.tb01782.x. [DOI] [PubMed] [Google Scholar]
- 8.Neumann R. Experimentelle Beitrage zur Lehre von dem täglichen Nahrungsbedarf des Menschen unter besonderer Berücksichtigung der notwendigen Eiweissmenge. Arch Hyg. 1902;45:1–87. [Google Scholar]
- 9.Bray GA. Obesity, a disorder of nutrient partitioning: the MONA LISA hypothesis. J Nutr. 1991;121:1146–1162. doi: 10.1093/jn/121.8.1146. [DOI] [PubMed] [Google Scholar]
- 10.Johnston M. Feasting, fasting and fermenting. Glucose sensing in yeast and other cells. Trends Genet. 1999;15:29–33. doi: 10.1016/s0168-9525(98)01637-0. [DOI] [PubMed] [Google Scholar]
- 11.Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- 12.Rossetti L. Perspective: hexosamines and nutrient sensing. Endocrinology. 2000;141:1922–1925. doi: 10.1210/endo.141.6.7566. [DOI] [PubMed] [Google Scholar]
- 13.McClain DA. Hexosamines as mediators of nutrient sensing and regulation in diabetes. J Diabetes Complications. 2002;16:72–80. doi: 10.1016/s1056-8727(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 14.Rossetti L, et al. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J Clin Invest. 1995;96:132–140. doi: 10.1172/JCI118013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkins M, et al. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest. 1997;99:2173–2182. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi CS, Lee FN, Youn JH. Free fatty acids induce peripheral insulin resistance without increasing muscle hexosamine pathway product levels in rats. Diabetes. 2001;50:418–424. doi: 10.2337/diabetes.50.2.418. [DOI] [PubMed] [Google Scholar]
- 17.Monauni T, et al. Effects of glucosamine infusion on insulin secretion and insulin action in humans. Diabetes. 2000;49:926–935. doi: 10.2337/diabetes.49.6.926. [DOI] [PubMed] [Google Scholar]
- 18.Pouwels MJ, et al. Short-term glucosamine infusion does not affect insulin sensitivity in humans. J Clin Endocrinol Metab. 2001;86:2099–2103. doi: 10.1210/jcem.86.5.7470. [DOI] [PubMed] [Google Scholar]
- 19.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, et al. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]