Abstract
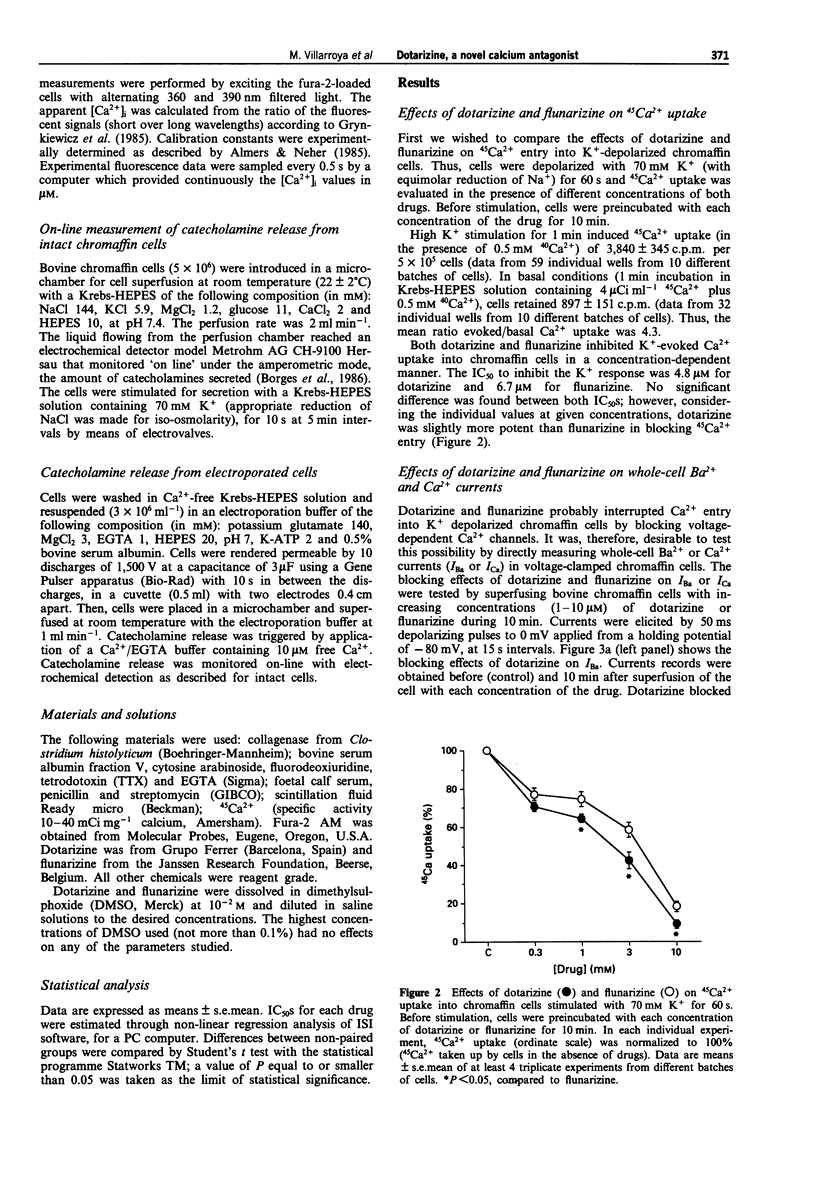
1. Dotarizine is a novel piperazine derivative structurally related to flunarizine that is currently being evaluated in clinical trials for its antimigraine and antivertigo effects. This clinical profile may be related to its Ca2+ antagonist properties. Therefore, the actions of both compounds as calcium antagonists were compared in bovine chromaffin cells. 2. Dotarizine and flunarizine blocked 45Ca2+ uptake into K+ depolarized chromaffin cells (70 mM K+/0.5 mM Ca2+ for 60 s) in a concentration-dependent manner, with IC50s of 4.8 and 6.7 microM, respectively. 3. Dotarizine and flunarizine also inhibited the whole-cell Ca2+ and Ba2+ currents (ICa, IBa) in voltage-clamped chromaffin cells, induced by depolarizing test pulses to 0 mV, during 50 ms, from a holding potential of -80 mV. Blockade exhibited IC50s of 4 microM for dotarizine and 2.2 microM for flunarizine. Dotarizine increased the rate of inactivation of ICa and IBa; inhibition of whole-cell currents was use-dependent. 4. Transient increases of the cytosolic Ca2+ concentration, [Ca2+]i, produced by K+ stimulation (70 mM K+ for 5 s) of single fura-2-loaded chromaffin cells, were also inhibited by dotarizine and flunarizine with IC50s of 1.2 and 0.6 microM, respectively. Upon washout of dotarizine, the [Ca2+]i increases recovered fully after 5-10 min. In contrast, the responses remained largely inhibited 10 min after washing out flunarizine. 5. Catecholamine release induced by K+ stimulation (10-s pulses of 70 mM) was inhibited by dotarizine with an IC50 of 2.6 microM and by flunarizine with an IC50 of 1.2 microM. The blocking effects of both compounds developed slowly, and was fully established after 20-30 min of superfusion.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albillos A., García A. G., Gandía L. omega-Agatoxin-IVA-sensitive calcium channels in bovine chromaffin cells. FEBS Lett. 1993 Dec 27;336(2):259–262. doi: 10.1016/0014-5793(93)80815-c. [DOI] [PubMed] [Google Scholar]
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Boarder M. R., Marriott D., Adams M. Stimulus secretion coupling in cultured chromaffin cells. Dependency on external sodium and on dihydropyridine-sensitive calcium channels. Biochem Pharmacol. 1987 Jan 1;36(1):163–167. doi: 10.1016/0006-2952(87)90394-7. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Cartheuser C. F., Brasó A., Sacristán A., Ortiz J. A. Effects of dotarizine on peripheral and pulmonary circulation and cardiac dynamics in dogs. Pharmacology. 1994 Mar;48(3):187–193. doi: 10.1159/000139178. [DOI] [PubMed] [Google Scholar]
- Ceña V., Nicolas G. P., Sanchez-Garcia P., Kirpekar S. M., Garcia A. G. Pharmacological dissection of receptor-associated and voltage-sensitive ionic channels involved in catecholamine release. Neuroscience. 1983 Dec;10(4):1455–1462. doi: 10.1016/0306-4522(83)90126-4. [DOI] [PubMed] [Google Scholar]
- De la Fuente M. T., Guantes J. M., Del Valle M., Garcia A. G. Mechanism of blockade by flunarizine of bovine adrenal catecholamine release. Eur J Pharmacol. 1992 Dec 15;229(2-3):189–196. doi: 10.1016/0014-2999(92)90554-h. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. History of calcium antagonists. Circ Res. 1983 Feb;52(2 Pt 2):I3–16. [PubMed] [Google Scholar]
- Gandía L., Albillos A., García A. G. Bovine chromaffin cells possess FTX-sensitive calcium channels. Biochem Biophys Res Commun. 1993 Jul 30;194(2):671–676. doi: 10.1006/bbrc.1993.1874. [DOI] [PubMed] [Google Scholar]
- Gandía L., Michelena P., de Pascual R., López M. G., García A. G. Different sensitivities to dihydropyridines of catecholamine release from cat and ox adrenals. Neuroreport. 1990 Oct;1(2):119–122. doi: 10.1097/00001756-199010000-00009. [DOI] [PubMed] [Google Scholar]
- García A. G., Sala F., Reig J. A., Viniegra S., Frías J., Fontériz R., Gandía L. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984 May 3;309(5963):69–71. doi: 10.1038/309069a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hans M., Illes P., Takeda K. The blocking effects of omega-conotoxin on Ca current in bovine chromaffin cells. Neurosci Lett. 1990 Jun 22;114(1):63–68. doi: 10.1016/0304-3940(90)90429-d. [DOI] [PubMed] [Google Scholar]
- Jiménez R. R., López M. G., Sancho C., Maroto R., García A. G. A component of the catecholamine secretory response in the bovine adrenal gland is resistant to dihydropyridines and omega-conotoxin. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1278–1283. doi: 10.1006/bbrc.1993.1355. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Akaike N. The low-threshold Ca current in isolated amygdaloid neurons in the rat. Brain Res. 1989 Sep 11;497(1):187–190. doi: 10.1016/0006-8993(89)90987-6. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Livett B. G. Adrenal medullary chromaffin cells in vitro. Physiol Rev. 1984 Oct;64(4):1103–1161. doi: 10.1152/physrev.1984.64.4.1103. [DOI] [PubMed] [Google Scholar]
- Michelena P., García-Pérez L. E., Artalejo A. R., García A. G. Separation between cytosolic calcium and secretion in chromaffin cells superfused with calcium ramps. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3284–3288. doi: 10.1073/pnas.90.8.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro M. A., López M. G., Gandía L., Michelena P., García A. G. Separation and culture of living adrenaline- and noradrenaline-containing cells from bovine adrenal medullae. Anal Biochem. 1990 Mar;185(2):243–248. doi: 10.1016/0003-2697(90)90287-j. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Miljanich G. P., Ramachandran J., Adams M. E. Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- Scheufler E., Peters T. Phosphatidylserine monolayers as models for drug uptake into membranes and tissue. Cell Biol Int Rep. 1990 Apr;14(4):381–388. doi: 10.1016/0309-1651(90)91207-k. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Akaike N. Calcium antagonist effects on low-threshold (T-type) calcium current in rat isolated hippocampal CA1 pyramidal neurons. J Pharmacol Exp Ther. 1991 Jan;256(1):169–175. [PubMed] [Google Scholar]
- Tejerina T., Chulia T., Gonzalez P. Effects of dotarizine on 45Ca2+ movements and contractile responses in vascular smooth muscle. Eur J Pharmacol. 1993 Aug 3;239(1-3):75–81. doi: 10.1016/0014-2999(93)90978-q. [DOI] [PubMed] [Google Scholar]
- Thomas P. G., Seelig J. Binding of the calcium antagonist flunarizine to phosphatidylcholine bilayers: charge effects and thermodynamics. Biochem J. 1993 Apr 15;291(Pt 2):397–402. doi: 10.1042/bj2910397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd P. A., Benfield P. Flunarizine. A reappraisal of its pharmacological properties and therapeutic use in neurological disorders. Drugs. 1989 Oct;38(4):481–499. doi: 10.2165/00003495-198938040-00002. [DOI] [PubMed] [Google Scholar]


