Abstract
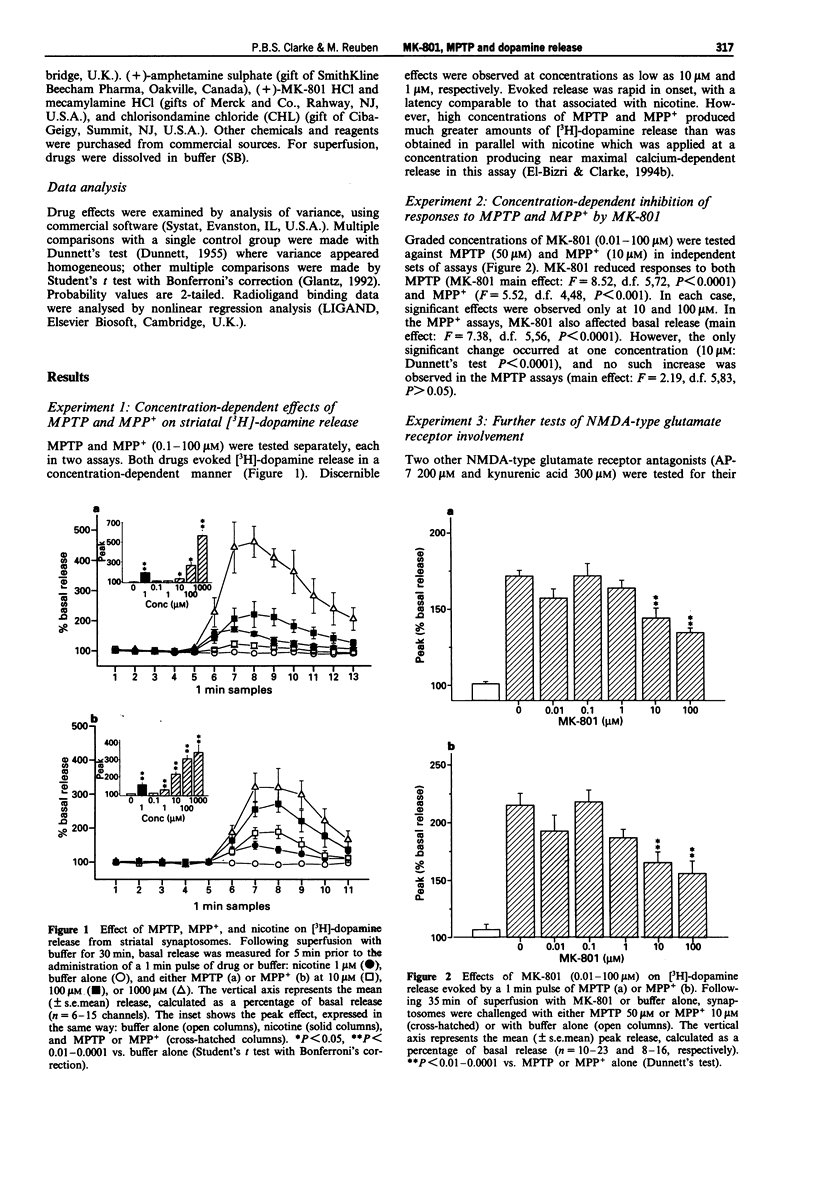
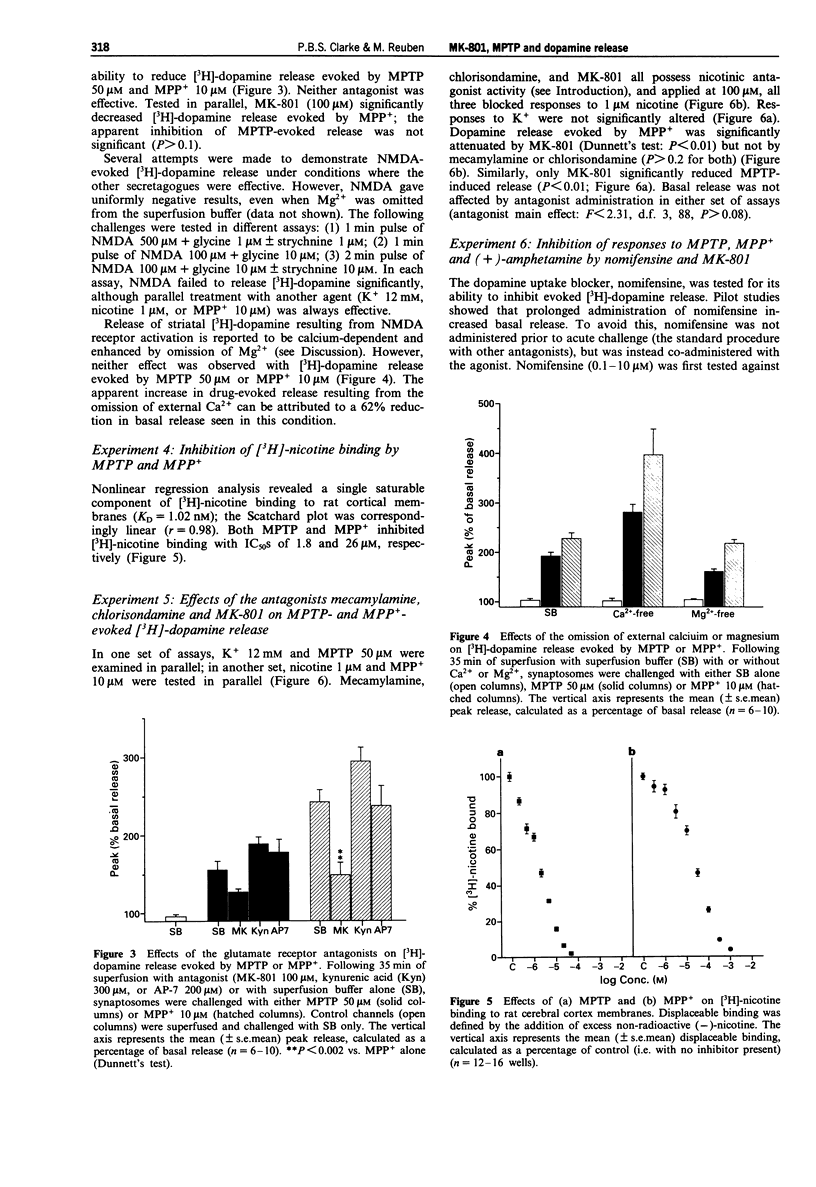
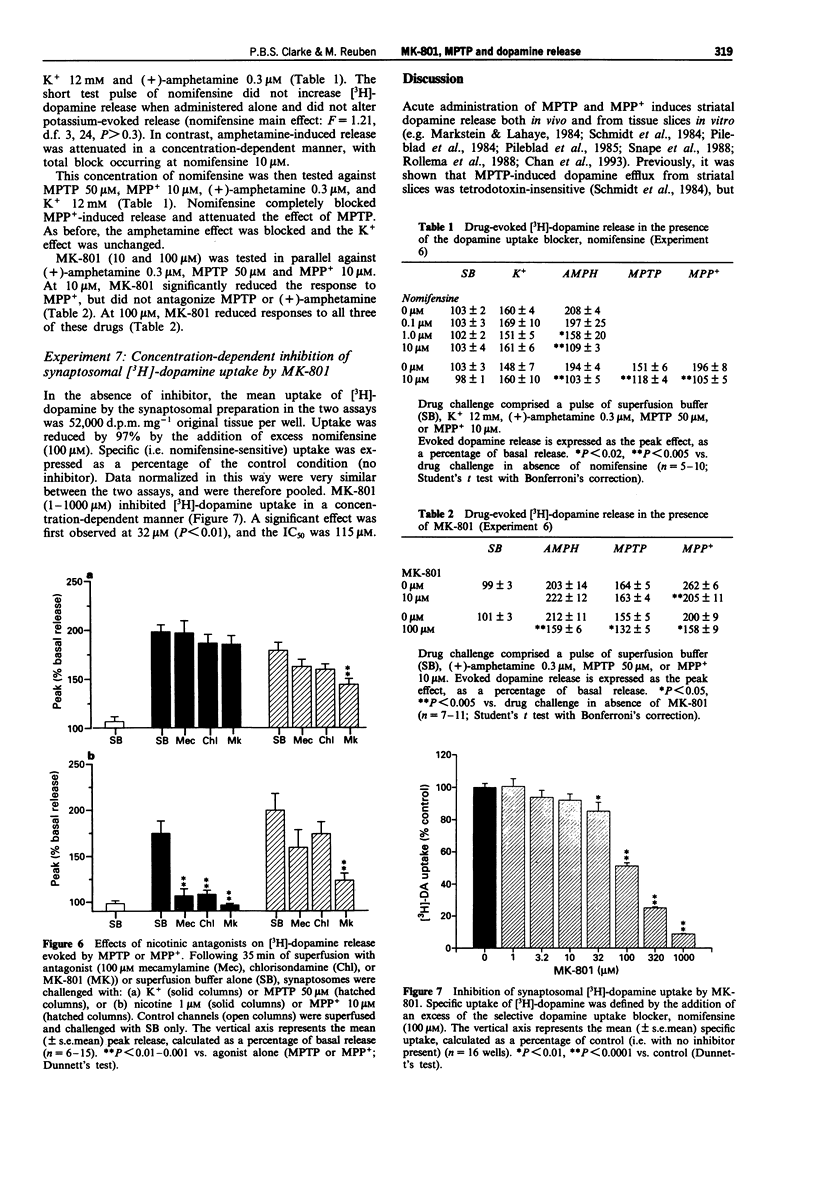
1. The NMDA-type glutamate receptor antagonist, dizocilpine (MK-801) can protect against neurotoxicity associated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and its principal metabolite, the 1-methyl-4-phenylpyridinium ion (MPP+). It has been suggested that these neurotoxic effects may be mediated by release of excitatory amino acids, but possible alternative mechanisms have been little investigated. 2. MPTP and MPP+ (0.1-1000 microM) were tested in superfused rat striatal synaptosomes preloaded with [3H]-dopamine. Both MPTP (10 microM and higher) and MPP+ (1 microM and higher) evoked an immediate and concentration-dependent release of [3H]-dopamine. The maximal effect exceeded that achievable with nicotine. For subsequent experiments, submaximal concentrations of MPTP (50 microM) and MPP+ (10 microM) were tested. 3. MK-801 (0.1-100 microM) inhibited responses to MPTP (50 microM) and MPP+ (10 microM) in a concentration-dependent manner. However, further tests of NMDA-type glutamate receptor involvement proved negative. Responses to MPTP or MPP+ were unaffected by the omission of Mg2+ or Ca2+ and were not reduced by the NMDA receptor antagonists, AP-7 (200 microM) and kynurenic acid (300 microM). In this assay, N-methyl-D-aspartate (even in the absence of Mg2+ and with added glycine and strychnine) did not evoked [3H]-dopamine release. 4. In crude membrane preparations of rat cerebral cortex, MPTP and MPP+ inhibited high-affinity [3H]-nicotine binding to nicotinic cholinoceptors (IC50 1.8 microM and 26 microM, respectively).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brouillet E., Beal M. F. NMDA antagonists partially protect against MPTP induced neurotoxicity in mice. Neuroreport. 1993 Apr;4(4):387–390. doi: 10.1097/00001756-199304000-00011. [DOI] [PubMed] [Google Scholar]
- Carboni S., Melis F., Pani L., Hadjiconstantinou M., Rossetti Z. L. The non-competitive NMDA-receptor antagonist MK-801 prevents the massive release of glutamate and aspartate from rat striatum induced by 1-methyl-4-phenylpyridinium (MPP+). Neurosci Lett. 1990 Sep 4;117(1-2):129–133. doi: 10.1016/0304-3940(90)90131-r. [DOI] [PubMed] [Google Scholar]
- Carter C. J., L'Heureux R., Scatton B. Differential control by N-methyl-D-aspartate and kainate of striatal dopamine release in vivo: a trans-striatal dialysis study. J Neurochem. 1988 Aug;51(2):462–468. doi: 10.1111/j.1471-4159.1988.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Chan P., DeLanney L. E., Irwin I., Langston J. W., Di Monte D. Rapid ATP loss caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mouse brain. J Neurochem. 1991 Jul;57(1):348–351. doi: 10.1111/j.1471-4159.1991.tb02134.x. [DOI] [PubMed] [Google Scholar]
- Chan P., Langston J. W., Di Monte D. A. MK-801 temporarily prevents MPTP-induced acute dopamine depletion and MPP+ elimination in the mouse striatum. J Pharmacol Exp Ther. 1993 Dec;267(3):1515–1520. [PubMed] [Google Scholar]
- Clarke P. B., Reuben M., el-Bizri H. Blockade of nicotinic responses by physostigmine, tacrine and other cholinesterase inhibitors in rat striatum. Br J Pharmacol. 1994 Mar;111(3):695–702. doi: 10.1111/j.1476-5381.1994.tb14793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow D. W., Jhamandas K. Characterization of L-glutamate action on the release of endogenous dopamine from the rat caudate-putamen. J Pharmacol Exp Ther. 1989 Feb;248(2):722–728. [PubMed] [Google Scholar]
- Desce J. M., Godeheu G., Galli T., Artaud F., Chéramy A., Glowinski J. L-glutamate-evoked release of dopamine from synaptosomes of the rat striatum: involvement of AMPA and N-methyl-D-aspartate receptors. Neuroscience. 1992;47(2):333–339. doi: 10.1016/0306-4522(92)90249-2. [DOI] [PubMed] [Google Scholar]
- Duchemin A. M., Gudehithlu K. P., Neff N. H., Hadjiconstantinou M. c-fos mRNA in mouse brain after MPTP treatment. Neurochem Int. 1992 Apr;20(3):281–287. doi: 10.1016/0197-0186(92)90042-p. [DOI] [PubMed] [Google Scholar]
- Finiels-Marlier F., Marini A. M., Williams P., Paul S. M. The N-methyl-D-aspartate antagonist MK-801 fails to protect dopaminergic neurons from 1-methyl-4-phenylpyridinium toxicity in vitro. J Neurochem. 1993 May;60(5):1968–1971. doi: 10.1111/j.1471-4159.1993.tb13431.x. [DOI] [PubMed] [Google Scholar]
- Hsu K. S., Fu W. M., Lin-Shiau S. Y. Studies on the neuromuscular blocking action of MPTP in the mouse phrenic nerve-diaphragm. Neuropharmacology. 1993 Jun;32(6):597–603. doi: 10.1016/0028-3908(93)90056-9. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson A. M., Fuxe K., Goldstein M. Differential effects of acute and chronic nicotine treatment on MPTP-(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced degeneration of nigrostriatal dopamine neurons in the black mouse. Clin Investig. 1992 Mar-Apr;70(3-4):232–238. doi: 10.1007/BF00184656. [DOI] [PubMed] [Google Scholar]
- Javitch J. A., D'Amato R. J., Strittmatter S. M., Snyder S. H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. M., Jeng Y. J. Pharmacological evidence for N-methyl-D-aspartate receptors on nigrostriatal dopaminergic nerve terminals. Can J Physiol Pharmacol. 1991 Oct;69(10):1416–1421. doi: 10.1139/y91-212. [DOI] [PubMed] [Google Scholar]
- Krebs M. O., Desce J. M., Kemel M. L., Gauchy C., Godeheu G., Cheramy A., Glowinski J. Glutamatergic control of dopamine release in the rat striatum: evidence for presynaptic N-methyl-D-aspartate receptors on dopaminergic nerve terminals. J Neurochem. 1991 Jan;56(1):81–85. doi: 10.1111/j.1471-4159.1991.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Kupsch A., Löschmann P. A., Sauer H., Arnold G., Renner P., Pufal D., Burg M., Wachtel H., ten Bruggencate G., Oertel W. H. Do NMDA receptor antagonists protect against MPTP-toxicity? Biochemical and immunocytochemical analyses in black mice. Brain Res. 1992 Oct 2;592(1-2):74–83. doi: 10.1016/0006-8993(92)91660-7. [DOI] [PubMed] [Google Scholar]
- Lange K. W., Löschmann P. A., Sofic E., Burg M., Horowski R., Kalveram K. T., Wachtel H., Riederer P. The competitive NMDA antagonist CPP protects substantia nigra neurons from MPTP-induced degeneration in primates. Naunyn Schmiedebergs Arch Pharmacol. 1993 Dec;348(6):586–592. doi: 10.1007/BF00167234. [DOI] [PubMed] [Google Scholar]
- Levi G., Raiteri M. Carrier-mediated release of neurotransmitters. Trends Neurosci. 1993 Oct;16(10):415–419. doi: 10.1016/0166-2236(93)90010-j. [DOI] [PubMed] [Google Scholar]
- Markey S. P., Johannessen J. N., Chiueh C. C., Burns R. S., Herkenham M. A. Intraneuronal generation of a pyridinium metabolite may cause drug-induced parkinsonism. Nature. 1984 Oct 4;311(5985):464–467. doi: 10.1038/311464a0. [DOI] [PubMed] [Google Scholar]
- Markstein R., Lahaye D. Neurochemical investigations in vitro with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in preparations of rat brain. Eur J Pharmacol. 1984 Nov 13;106(2):301–311. doi: 10.1016/0014-2999(84)90717-9. [DOI] [PubMed] [Google Scholar]
- Marshall J. F., O'Dell S. J., Weihmuller F. B. Dopamine-glutamate interactions in methamphetamine-induced neurotoxicity. J Neural Transm Gen Sect. 1993;91(2-3):241–254. doi: 10.1007/BF01245234. [DOI] [PubMed] [Google Scholar]
- Namura I., Douillet P., Sun C. J., Pert A., Cohen R. M., Chiueh C. C. MPP+ (1-methyl-4-phenylpyridine) is a neurotoxin to dopamine-, norepinephrine- and serotonin-containing neurons. Eur J Pharmacol. 1987 Apr 7;136(1):31–37. doi: 10.1016/0014-2999(87)90775-8. [DOI] [PubMed] [Google Scholar]
- Palombo E., Porrino L. J., Crane A. M., Bankiewicz K. S., Kopin I. J., Sokoloff L. Cerebral metabolic effects of monoamine oxidase inhibition in normal and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine acutely treated monkeys. J Neurochem. 1991 May;56(5):1639–1646. doi: 10.1111/j.1471-4159.1991.tb02062.x. [DOI] [PubMed] [Google Scholar]
- Pileblad E., Fornstedt B., Clark D., Carlsson A. Acute effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on dopamine metabolism in mouse and rat striatum. J Pharm Pharmacol. 1985 Oct;37(10):707–711. doi: 10.1111/j.2042-7158.1985.tb04947.x. [DOI] [PubMed] [Google Scholar]
- Pileblad E., Nissbrandt H., Carlsson A. Biochemical and functional evidence for a marked dopamine releasing action of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (NMPTP) in mouse brain. J Neural Transm. 1984;60(3-4):199–203. doi: 10.1007/BF01249093. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Cerrito F., Cervoni A. M., Levi G. Dopamine can be released by two mechanisms differentially affected by the dopamine transport inhibitor nomifensine. J Pharmacol Exp Ther. 1979 Feb;208(2):195–202. [PubMed] [Google Scholar]
- Ramoa A. S., Alkondon M., Aracava Y., Irons J., Lunt G. G., Deshpande S. S., Wonnacott S., Aronstam R. S., Albuquerque E. X. The anticonvulsant MK-801 interacts with peripheral and central nicotinic acetylcholine receptor ion channels. J Pharmacol Exp Ther. 1990 Jul;254(1):71–82. [PubMed] [Google Scholar]
- Rapier C., Lunt G. G., Wonnacott S. Stereoselective nicotine-induced release of dopamine from striatal synaptosomes: concentration dependence and repetitive stimulation. J Neurochem. 1988 Apr;50(4):1123–1130. doi: 10.1111/j.1471-4159.1988.tb10582.x. [DOI] [PubMed] [Google Scholar]
- Ricaurte G. A., Langston J. W., DeLanney L. E., Irwin I., Brooks J. D. Dopamine uptake blockers protect against the dopamine depleting effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse striatum. Neurosci Lett. 1985 Sep 6;59(3):259–264. doi: 10.1016/0304-3940(85)90141-7. [DOI] [PubMed] [Google Scholar]
- Rollema H., Kuhr W. G., Kranenborg G., De Vries J., Van den Berg C. MPP+-induced efflux of dopamine and lactate from rat striatum have similar time courses as shown by in vivo brain dialysis. J Pharmacol Exp Ther. 1988 Jun;245(3):858–866. [PubMed] [Google Scholar]
- Romm E., Lippiello P. M., Marks M. J., Collins A. C. Purification of L-[3H]nicotine eliminates low affinity binding. Life Sci. 1990;46(13):935–943. doi: 10.1016/0024-3205(90)90095-9. [DOI] [PubMed] [Google Scholar]
- Santiago M., Venero J. L., Machado A., Cano J. In vivo protection of striatum from MPP+ neurotoxicity by N-methyl-D-aspartate antagonists. Brain Res. 1992 Jul 24;586(2):203–207. doi: 10.1016/0006-8993(92)91628-r. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Matsuda L. A., Gibb J. W. In vitro release of tritiated monoamines from rat CNS tissue by the neurotoxic compound 1-methyl-phenyl-tetrahydropyridine. Eur J Pharmacol. 1984 Aug 17;103(3-4):255–260. doi: 10.1016/0014-2999(84)90485-0. [DOI] [PubMed] [Google Scholar]
- Scotcher K. P., Irwin I., DeLanney L. E., Langston J. W., Di Monte D. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on ATP levels of mouse brain synaptosomes. J Neurochem. 1990 Apr;54(4):1295–1301. doi: 10.1111/j.1471-4159.1990.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Sirinathsinghji D. J., Heavens R. P., McBride C. S. Dopamine-releasing action of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 1-methyl-4-phenylpyridine (MPP+) in the neostriatum of the rat as demonstrated in vivo by the push-pull perfusion technique: dependence on sodium but not calcium ions. Brain Res. 1988 Mar 8;443(1-2):101–116. doi: 10.1016/0006-8993(88)91603-4. [DOI] [PubMed] [Google Scholar]
- Snape B. M., Pileblad E., Ekman A., Magnusson T., Carlsson A., Engel J. The effects of 1-methyl-4-phenylpyridinium ion (MPP+) on the efflux and metabolism of endogenous dopamine in rat striatal slices. J Pharm Pharmacol. 1988 Sep;40(9):620–626. doi: 10.1111/j.2042-7158.1988.tb05321.x. [DOI] [PubMed] [Google Scholar]
- Snell L. D., Johnson K. M. Characterization of the inhibition of excitatory amino acid-induced neurotransmitter release in the rat striatum by phencyclidine-like drugs. J Pharmacol Exp Ther. 1986 Sep;238(3):938–946. [PubMed] [Google Scholar]
- Snell L. D., Yi S. J., Johnson K. M. Comparison of the effects of MK-801 and phencyclidine on catecholamine uptake and NMDA-induced norepinephrine release. Eur J Pharmacol. 1988 Jan 12;145(2):223–226. doi: 10.1016/0014-2999(88)90235-x. [DOI] [PubMed] [Google Scholar]
- Sonsalla P. K., Nicklas W. J., Heikkila R. E. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989 Jan 20;243(4889):398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- Sonsalla P. K., Zeevalk G. D., Manzino L., Giovanni A., Nicklas W. J. MK-801 fails to protect against the dopaminergic neuropathology produced by systemic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice or intranigral 1-methyl-4-phenylpyridinium in rats. J Neurochem. 1992 May;58(5):1979–1982. doi: 10.1111/j.1471-4159.1992.tb10081.x. [DOI] [PubMed] [Google Scholar]
- Srivastava R., Brouillet E., Beal M. F., Storey E., Hyman B. T. Blockade of 1-methyl-4-phenylpyridinium ion (MPP+) nigral toxicity in the rat by prior decortication or MK-801 treatment: a stereological estimate of neuronal loss. Neurobiol Aging. 1993 Jul-Aug;14(4):295–301. doi: 10.1016/0197-4580(93)90114-q. [DOI] [PubMed] [Google Scholar]
- Storey E., Hyman B. T., Jenkins B., Brouillet E., Miller J. M., Rosen B. R., Beal M. F. 1-Methyl-4-phenylpyridinium produces excitotoxic lesions in rat striatum as a result of impairment of oxidative metabolism. J Neurochem. 1992 May;58(5):1975–1978. doi: 10.1111/j.1471-4159.1992.tb10080.x. [DOI] [PubMed] [Google Scholar]
- Tabatabaei A., Perry T. L., Hansen S., Krieger C. Partial protective effect of MK-801 on MPTP-induced reduction of striatal dopamine in mice. Neurosci Lett. 1992 Jul 20;141(2):192–194. doi: 10.1016/0304-3940(92)90892-b. [DOI] [PubMed] [Google Scholar]
- Tipton K. F., Singer T. P. Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J Neurochem. 1993 Oct;61(4):1191–1206. doi: 10.1111/j.1471-4159.1993.tb13610.x. [DOI] [PubMed] [Google Scholar]
- Turski L., Bressler K., Rettig K. J., Löschmann P. A., Wachtel H. Protection of substantia nigra from MPP+ neurotoxicity by N-methyl-D-aspartate antagonists. Nature. 1991 Jan 31;349(6308):414–418. doi: 10.1038/349414a0. [DOI] [PubMed] [Google Scholar]
- Vezzani A., Serafini R., Stasi M. A., Caccia S., Conti I., Tridico R. V., Samanin R. Kinetics of MK-801 and its effect on quinolinic acid-induced seizures and neurotoxicity in rats. J Pharmacol Exp Ther. 1989 Apr;249(1):278–283. [PubMed] [Google Scholar]
- Vyas I., Heikkila R. E., Nicklas W. J. Studies on the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: inhibition of NAD-linked substrate oxidation by its metabolite, 1-methyl-4-phenylpyridinium. J Neurochem. 1986 May;46(5):1501–1507. doi: 10.1111/j.1471-4159.1986.tb01768.x. [DOI] [PubMed] [Google Scholar]
- Wamil A. W., McLean M. J. Use-, concentration- and voltage-dependent limitation by MK-801 of action potential firing frequency in mouse central neurons in cell culture. J Pharmacol Exp Ther. 1992 Jan;260(1):376–383. [PubMed] [Google Scholar]
- Wang J. K. Presynaptic glutamate receptors modulate dopamine release from striatal synaptosomes. J Neurochem. 1991 Sep;57(3):819–822. doi: 10.1111/j.1471-4159.1991.tb08224.x. [DOI] [PubMed] [Google Scholar]
- Weihmuller F. B., O'Dell S. J., Marshall J. F. MK-801 protection against methamphetamine-induced striatal dopamine terminal injury is associated with attenuated dopamine overflow. Synapse. 1992 Jun;11(2):155–163. doi: 10.1002/syn.890110209. [DOI] [PubMed] [Google Scholar]
- Willis C. L., Brazell C., Foster A. C. Plasma and CSF levels of dizocilpine (MK-801) required for neuroprotection in the quinolinate-injected rat striatum. Eur J Pharmacol. 1991 Apr 24;196(3):285–290. doi: 10.1016/0014-2999(91)90441-r. [DOI] [PubMed] [Google Scholar]
- Wilson J. A., Doyle T. J., Lau Y. S. MPTP, MPDP+ and MPP+ cause decreases in dopamine content in mouse brain slices. Neurosci Lett. 1990 Jan 1;108(1-2):213–218. doi: 10.1016/0304-3940(90)90733-p. [DOI] [PubMed] [Google Scholar]
- Wilson J. A., Lau Y. S., Gleeson J. G., Wilson J. S. The action of MPTP on synaptic transmission is affected by changes in Ca2+ concentrations. Brain Res. 1991 Feb 15;541(2):342–346. doi: 10.1016/0006-8993(91)91035-y. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A. Sites for antagonism on the N-methyl-D-aspartate receptor channel complex. Annu Rev Pharmacol Toxicol. 1991;31:401–425. doi: 10.1146/annurev.pa.31.040191.002153. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Brain nicotine binding sites. Hum Toxicol. 1987 Sep;6(5):343–353. doi: 10.1177/096032718700600502. [DOI] [PubMed] [Google Scholar]
- de Belleroche J. S., Bradford H. F. Presynaptic control of the synthesis and release of dopamine from striatal synaptosomes: a comparison between the effects of 5-hydroxytryptamine, acetylcholine, and glutamate. J Neurochem. 1980 Nov;35(5):1227–1234. doi: 10.1111/j.1471-4159.1980.tb07879.x. [DOI] [PubMed] [Google Scholar]
- el-Bizri H., Clarke P. B. Blockade of nicotinic receptor-mediated release of dopamine from striatal synaptosomes by chlorisondamine and other nicotinic antagonists administered in vitro. Br J Pharmacol. 1994 Feb;111(2):406–413. doi: 10.1111/j.1476-5381.1994.tb14749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Bizri H., Clarke P. B. Regulation of nicotinic receptors in rat brain following quasi-irreversible nicotinic blockade by chlorisondamine and chronic treatment with nicotine. Br J Pharmacol. 1994 Nov;113(3):917–925. doi: 10.1111/j.1476-5381.1994.tb17080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


