Abstract
Heparin has been used clinically as an anticoagulant and antithrombotic agent for over 60 years. Here we show that the potent anti-inflammatory property of heparin results primarily from blockade of P-selectin and L-selectin. Unfractionated heparin and chemically modified analogs were tested as inhibitors of selectin binding to immobilized sialyl LewisX and of cell adhesion to immobilized selectins or thrombin-activated endothelial cells. Compared with unfractionated heparin, the modified heparinoids had inhibitory activity in this general order: over–O-sulfated heparin > heparin > 2-O,3-O-desulfated ≥ N-desulfated/N-acetylated heparin ≥ carboxyl-reduced heparin ≥ N-,2-O,3-O-desulfated heparin >> 6-O-desulfated heparin. The heparinoids also showed similar differences in their ability to inhibit thioglycollate-induced peritonitis and oxazolone-induced delayed-type hypersensitivity. Mice deficient in P- or L-selectins showed impaired inflammation, which could be further reduced by heparin. However, heparin had no additional effect in mice deficient in both P- and L-selectins. We conclude that (a) heparin’s anti-inflammatory effects are mainly mediated by blocking P- and L-selectin–initiated cell adhesion; (b) the sulfate groups at C6 on the glucosamine residues play a critical role in selectin inhibition; and (c) some non-anticoagulant forms of heparin retain anti-inflammatory activity. Such analogs may prove useful as therapeutically effective inhibitors of inflammation.
Introduction
The recruitment of leukocytes from the blood and lymphatic systems into tissues facilitates a successful host response to tissue injury and pathogen invasion. When these processes go awry, they can contribute to the pathophysiology of acute and chronic inflammatory disease. Members of the selectin family of adhesion receptors (E, P, and L) mediate the initial adhesive events that direct the movement of leukocytes across the endothelium in inflamed tissues (reviewed in refs. 1–15). All three selectins contain an amino-terminal, calcium-dependent carbohydrate recognition domain that binds to carbohydrate ligands on cells. P-selectin is rapidly mobilized to the surface of endothelial cells or platelets exposed to thrombin or histamine, and L-selectin is expressed constitutively on circulating leukocytes. In contrast, agents such as IL-1, TNF-α, and endotoxin induce E-selectin specifically in endothelial cells several hours after activation. P-selectin and L-selectin appear to mediate the initial events in cellular infiltration due to their rapid appearance on activated endothelium and constitutive expression on leukocytes, respectively.
All three selectins bind to sialylated, fucosylated carbohydrate antigens related to sialyl LewisX [SLeX, Neu5Acα2,3Galβ1,4(Fucα1,3)GlcNAcβ-] (10, 11). SLeX determinants have been detected on specific selectin ligands, such as P-selectin glycoprotein ligand-1 (PSGL-1) and E-selectin ligand-1 (ESL-1), and on glycosylation-dependent cell adhesion molecule-1 (GlyCAM-1). These interactions appear to involve sulfate residues either on the carbohydrate or on the protein as sulfated tyrosine residues in proximity to the carbohydrate chain (12). Genetic data from mutant mice and inborn errors in humans substantiate the requirement of the glycosylated ligand for selectin function in vivo (13–15).
Several studies have shown that heparin and heparan sulfate can be recognized by L-selectin and P-selectin (16). Heparin and heparin-like oligosaccharides can inhibit L-selectin or P-selectin binding to SLeX-related compounds or to SLeX determinants on HL-60 cells at concentrations lower than those required for inhibition by SLeX itself (17–21). Injected heparin also affects the inflammatory response (22), causes leukocytosis (23–25), and attenuates tumor metastasis in mice by inhibiting P-selectin–mediated interactions of platelets with carcinoma cell-surface mucin ligands (26). These studies suggest that the anti-inflammatory effects of heparin are due to a blockade of P- and L-selectins, but direct evidence for this mode of action in vivo is lacking.
Heparin and heparan sulfate consist of repeating disaccharide units containing D-glucuronic acid (GlcA) or L-iduronic acid (IdoA) and a glucosamine residue that is either N-sulfated (GlcNS), N-acetylated (GlcNAc), or, occasionally, unsubstituted (GlcNH2) (27). The disaccharides may be further sulfated at C6 or C3 of the glucosamine residues and C2 of the uronic acid residues. The potent anticoagulant activity of heparin depends on a specific arrangement of sulfated sugar units and uronic acid epimers, which form a binding site for antithrombin (27). Much less information is available about the specific oligosaccharide structures in heparin that interact with P- and L-selectins (18, 21, 28). Thus, we also sought to understand the relationship of heparin fine structure to its interaction with these selectins.
In the present study, various chemically-modified heparin derivatives (heparinoids) were evaluated for their ability to block P- and L-selectin–dependent interactions with sialylated, fucosylated ligands in vitro and to block inflammation in mice. The results show that the inhibitory properties of heparin depend critically on 6-O-sulfated glucosamine residues. Furthermore, studies of selectin-deficient mice indicate that heparin interactions with P- and L-selectin can completely account for its anti-inflammatory activity in vivo. Together, the data indicate that non-anticoagulant forms of heparin that bind to P- or L-selectin may prove useful for treating inflammatory disease.
Methods
Heparin and its derivatives.
Porcine intestinal heparin (Mr = 12,000–15,000) was kindly provided by Patrick Shaklee (Scientific Protein Laboratories Inc., Milwaukee, Wisconsin, USA). N-desulfated/N-acetylated heparin (NDS-heparin) (29, 30), oversulfated heparin (OS-heparin) (31), and carboxyl-reduced heparin (CR-heparin) (32) were obtained from Glycomed Inc. (Alameda, California, USA). 2-O,3-O-desulfated heparin (2/3DS-heparin) was prepared according to the method of Fryer et al. (33). Completely 6-O-desulfated heparin (6DS-heparin) was produced by regioselective hydrolysis with N-methyl-N-(trimethylsilyl)trifluoroacetamide (34) and compared with a standard sample kindly provided by Yutaka Kariya (Seikagaku Corp., Tokyo, Japan). This preparation contained about 20% less 2-O-sulfate groups. The anticoagulant activity of heparin and modified heparinoids was analyzed by amidolytic anti–factor Xa assay (35). All samples tested negative for endotoxin using the Limulus test.
13C-NMR experiments were carried out at 80°C in D2O (sample volumes of 0.6–0.7 ml in 5-mm tubes; Wilmad Labglass, Buena, New Jersey, USA) on a Unity Inova 500 spectrometer (Varian Inc., Walnut Creek, California, USA). A 5-mm broadband observe-1H decoupling probe was used for all experiments. A SUN Microsystems (Santa Clara, California, USA) 4/330 computer running Varian’s VNMR software (version 4.1) controlled data acquisition. DEPT135 data sets were collected with the delay between pulses set to 3.6 ms corresponding to a J (C-H) value of 140 Hz, and the final proton pulse set to 135° to distinguish CH2 from all other carbon signals. Selected NMR spectra for the chemically synthesized 6DS-heparin were interpreted by comparison of the chemical shifts with those obtained with standard heparin.
Disaccharide composition was determined by complete depolymerization of the chains by hydrazinolysis, nitrous acid cleavage, and borotritide reduction, followed by reverse-phase ion-pairing chromatography using a Hi-Chrom S5 ODS C18 column (4.6 × 50 mm; Regis Technologies Inc., Morton Grove, Illinois, USA) as reported previously (36). Individual peaks were identified by comparison with standard disaccharides from commercial heparin and with published results. As reported previously, complete 6-O-desulfation was accompanied by partial loss of 2-O-sulfate groups on uronic acids (34).
Mice.
Wild-type, P-selectin–deficient (P–/–), and L-selectin–deficient (L–/–) mice bred on C57BL/6 background (6–8 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). The combined P-selectin– and L-selectin–deficient mice (PL–/–) were kindly provided by Richard O. Hynes (Massachusetts Institute of Technology, Cambridge, Massachusetts, USA) (37) and bred at the University of California, San Diego, in conformance with the university’s guidelines for the care and use of laboratory animals. Mice were weaned at 3 weeks, maintained on a 12-hour light-dark cycle, and fed water and standard rodent chow ad libitum.
Binding assays.
Inhibition of selectin-SLeX binding by the heparinoids was done by coating sterile polystyrene 96-well ELISA plates (Corning Inc., Corning, New York, USA) at 4°C overnight with 200 ng of polyacrylamide-SLeX (PAA-SLeX; Glycotech Corp., Rockville, Maryland, USA) in 100 μl of 50 mM sodium bicarbonate buffer, pH 9.5. Plates were blocked for 3 hours at 4°C with 200 μl/well of assay buffer containing 20 mM N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulfonic acid], pH 7.45, 125 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, and 1% protease-free BSA (Pentex; Miles Inc., Kankakee, Illinois, USA). Recombinant selectin-Ig chimeras were prepared as described previously (19) and were preincubated at 4°C for about 1 hour with peroxidase-conjugated goat anti-human IgG (1:1,000 dilution in assay buffer; Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA). The final selectin-Ig concentrations were 2.7, 1.9, and 5.0 μg/ml for E-, L-, and P-selectin, respectively. The selectin-Ig/secondary antibody stock was aliquoted into tubes containing heparin/analog, buffer (positive control), 10 mM sodium EDTA (negative control), anti–P-selectin, or anti–E-selectin adhesion-blocking mAb (1 μg; Pharmingen, San Diego, California, USA). The solutions (100 μl) were preincubated at 4°C for 30 minutes and added to ELISA plates. After 4 hours at 4°C, the plates were washed three times, followed by development with 2 μg/ml O-phenylenediamine dihydrochloride, 50 mM sodium citrate/sodium phosphate buffer, pH 5.2, and 0.03% H2O2. After 10 minutes, the peroxidase reaction was quenched by adding 50 μl of 4 M H2SO4. The absorbance at 492 nm was recorded using a microplate reader (Molecular Devices Inc., Menlo Park, California, USA) equipped with SOFTmax software (Molecular Devices Inc.). All the raw data were converted into percentages for comparative purposes using the formula: % of maximum = [(average of duplicates) – (negative control)]/[(positive control) – (negative control)] × 100.
U937 cell binding to immobilized E-, L-, or P-selectin.
The ability of heparin and the modified heparins to inhibit the adhesion of U937 cells to immobilized P-, L-, or E-selectins was examined by coating each well of a 96-well ELISA plate at 4°C overnight with 8 μg/ml of Protein A (Sigma-Aldrich, St. Louis, Missouri, USA) in 50 mM carbonate buffer, pH 9.4. After blocking the plate with 1% BSA in PBS, P-selectin (1 μg/well), L-selectin (5 μg/well), or E-selectin (1 μg/well) chimeras were added. After 1 hour, the wells were washed with blocking solution. U937 cells (CRL 1593.2; American Type Culture Collection, Rockville, Maryland, USA), were grown in RPMI-1640 medium (GIBCO BRL; Life Technologies Inc., Grand Island, New York, USA) containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin in an atmosphere of 5% CO2 in air and 100% relative humidity. The cells were fluorescently labeled with 10 μM Calcein AM (Molecular Probes Inc., Eugene, Oregon, USA) in RPMI-1640 medium containing 2.5% FBS for 30 minutes at 37°C. The cells were collected by low-speed centrifugation, washed three times with RPMI-1640 medium, and resuspended at a density of 2 × 106 cells/ml in medium without FBS. Heparin and heparin analogs, or 10 mM sodium EDTA, anti–P-selectin, or anti–E-selectin adhesion-blocking mAb (1 μg) in ELISA buffer, were added at 50 μl/well, and then the fluorescently labeled U937 cell suspension (50 μl/well) was added and incubated for 30 minutes at room temperature. Nonadherent cells were removed by rinsing the plates three times with PBS, and the number of adherent cells was quantified by measuring the fluorescence intensity (CytoFluor II; PerSeptive Biosystems, Framingham, Massachusetts, USA; excitation wavelength at 485 nm) after lysis of the cells with 2% Triton X-100 in 0.1 M Tris-HCl, pH 9.5. All the raw data were converted to relative fluorescence intensity for comparative purposes, using the formula given above for the ELISA.
Adherence to thrombin-stimulated human lung microvascular endothelial cells.
P-selectin–mediated U937 cell adhesion to human lung microvascular endothelial cells was measured as described previously (38). Cryopreserved human lung microvascular endothelial cells at passage 4–6 were maintained in complete medium (EGM-2-MV Bullet Kit; BioWhittaker Inc., Walkersville, Maryland, USA) at 37°C under an atmosphere of 5% CO2 and 100% relative humidity. The cells were seeded into 96-well flat-bottom cell culture plates at 2 × 104 cells per well, and a confluent monolayer was obtained within 2 days. The cells were then stimulated by adding 10 units per ml of human thrombin (Sigma-Aldrich) for 20 minutes. After washing once with warm RPMI-1640 medium, serially diluted heparin and analogs, 10 mM sodium EDTA, or anti–P-selectin adhesion-blocking mAb (1 μg) were added (50 μl/well). Control cells were incubated in ELISA assay buffer alone. U937 cells were fluorescently labeled as described above and added in 50 μl of ELISA buffer (105 per well) containing the heparinoids. After 20 minutes at room temperature, the wells were gently washed three times with PBS. Bound U937 cells were quantified by measuring the relative fluorescence intensity as described above.
Thioglycollate-induced peritoneal inflammation.
Mice were injected intraperitoneally with 2 ml of 3% thioglycollate broth (lot no. 54H4607; Sigma-Aldrich) or sterile pyrogen-free saline (GIBCO BRL; Life Technologies Inc.). Five minutes later, the animals received intravenous injections of 0.2 ml sterile pyrogen-free saline with and without heparin or analogs (0.5 or 1.25 mg/mouse). Mice were sacrificed after 3 hours, and the peritoneal cavities were lavaged with 8 ml of ice-cold PBS containing 3 mM EDTA to prevent clotting. Peritoneal cells were counted (Coulter Counter; Coulter Corp., Miami, Florida, USA). The cells were also stained for 30 minutes at 4°C with FITC-conjugated rat anti–mouse Gr-1 mAb (Pharmingen) diluted in PBS containing 2.5% FBS. After washing them three times with PBS, we counted the neutrophils on FACScan (Becton Dickinson Immunocytometry Systems, San Jose, California, USA) by gating the cells expressing a high level of Gr-1 antigen (39).
Allergic (delayed-type hypersensitivity) contact dermatitis.
Groups of five to eight animals per experimental set were sensitized with 100 μl of 2% oxazolone dissolved in acetone/olive oil (4:1, vol/vol) applied topically to the shaved abdominal skin. Allergic contact dermatitis (ACD), a form of delayed-type hypersensitivity (DTH), was elicited 5 days later by challenging the mice with 20 μl of 2% oxazolone in acetone/olive oil, administered topically to each side of the right ear (10 μl). The vehicle was applied in the same manner to each side of the left ear as a control. The thickness of the ear was measured before and 24 hours after challenge using a Mitutoyo engineer’s micrometer (Mitutoyo Corp., Auburn, Illinois, USA). The ACD reaction is presented as the increment of ear swelling after challenge expressed as the mean ± SE. The heparinoids (1mg/mouse in 100 μl saline) were administrated by intravenous injection within 30 minutes after antigen challenge.
Results
The inhibitory effects of intact and chemically modified heparin on selectin-SLeX interactions.
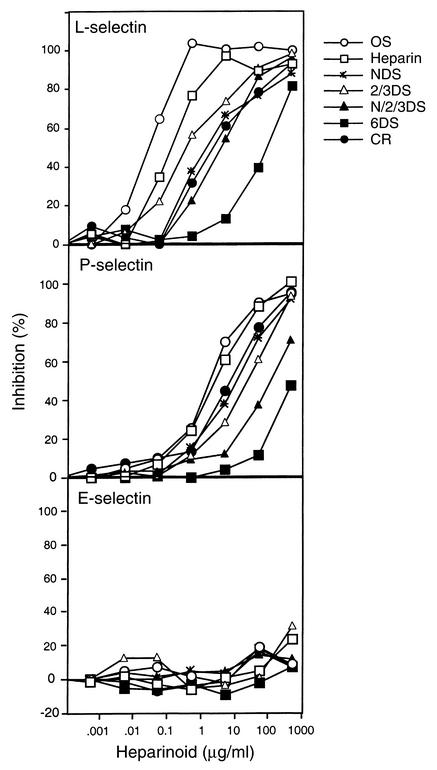
A series of modified derivatives was prepared, including CR-heparin, OS-heparin, NDS-heparin, 2/3DS-heparin, N-,2-O,3-O-desulfated heparin (N/2/3DS-heparin), and 6DS-heparin. Representative disaccharides of each type of heparinoid are given in Figure 1. Interaction with each selectin was inferred by measuring the ability of the heparinoids to block binding of L-, P-, and E-selectin–Ig chimeras to immobilized PAA-SLeX (Figure 2). Heparin inhibited the binding of L- and P-selectin–Ig chimera to PAA-SLeX but had no effect on E-selectin–Ig, in accordance with previous findings (18, 19, 21). Inhibition occurred across a wide concentration range, which likely reflects the structural heterogeneity inherently present in heparin (40). The residual binding at high concentrations was comparable to the level observed in the presence of 5 mM EDTA or with blocking antibodies to the selectins (∼90% inhibition), indicating that it is nonspecific.
Figure 1.
Representative disaccharide units of heparin and the various heparinoids. Each disaccharide illustrates a characteristic unit in the indicated preparation and does not represent the overall structure of the chains. X = H or SO3–.
Figure 2.
Inhibition of selectin-Ig binding to immobilized PAA-SLeX. Inhibition curves were generated using OS-heparin (open circles), heparin (open squares), NDS-heparin (asterisks), 2/3DS-heparin (open triangles), N/2/3DS-heparin (filled triangles), 6DS-heparin (filled squares), and CR-heparin (filled circles) (see Methods). Inclusion of a mAb to P-selectin, E-selectin, or EDTA blocked binding by more than 90%. Each point represents the average of duplicate determinations, and the data are representative of three or four separate experiments.
The various heparinoids differed significantly in their potency. Analysis of dose-response curves for native heparin yielded IC50 values of 0.1 and 2.5 μg/ml for blocking L- and P-selectins, respectively. OS-heparin was more potent (IC50 values of 0.01 and 1.8 μg/ml, respectively), whereas 6DS-heparin was least effective (IC50 values of 85 and 450 μg/ml, respectively). The other heparinoids gave intermediate levels of inhibition (Figure 2 and Table 1). Removal of the N-,2-O– and 3-O–linked sulfate groups or reduction of the carboxyl groups had the least effect. In general, higher concentrations were needed to inhibit P-selectin than to inhibit L-selectin, presumably reflecting the difference in affinity of the chimeras for clustered SLeX presented on the polyacrylamide resin.
Table 1.
Anticoagulant activities and inhibitory properties of heparin and its derivatives
Heparin inhibits adhesion of U937 cells.
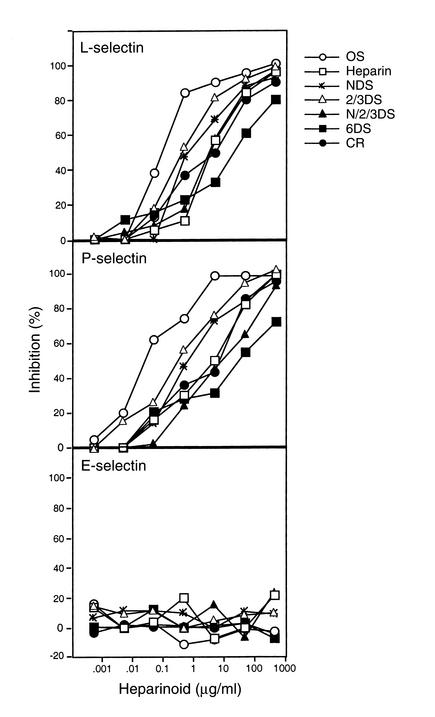
U937 cells express PSGL-1 (41), a natural ligand for P-selectin and L-selectin. As shown in Figure 3a, the various heparinoids also blocked adhesion of the cells to immobilized L-selectin in a concentration-dependent manner. Similar results were obtained with immobilized P-selectin (Figure 3b), but the compounds had no effect on cell adhesion to E-selectin (Figure 3c). The relative inhibitory efficacy of the heparinoids was similar to that observed in the competition assays (Figure 2), with OS-heparin exhibiting increased inhibitory activity compared with native heparin, and 6DS-heparin showing the lowest activity. In general, cell adhesion to P-selectin mediated by PSGL-1 on U937 cells was more sensitive to heparin inhibition than was binding of the selectin to PAA-SLeX, which may reflect differences in the density or arrangement of these ligands.
Figure 3.
Effects of heparinoids on selectin-dependent cell adhesion. Adhesion of U937 cells to immobilized selectin-Ig chimeras was measured (see Methods). Inclusion of a mAb to P-selectin, E-selectin, or EDTA blocked binding by more than 90%. Each point represents the average of duplicate determinations, and the data are representative of three or four separate experiments.
Since selectins normally mediate cell-cell contact, we also examined the effect of the heparinoids on adhesion of U937 cells to cultured human lung microvascular endothelial cells (Figure 4). P-selectin expression was induced by brief incubation with human thrombin (42), which increased the level of adhesion of U937 cells approximately threefold over the control. Inclusion of a mAb to P-selectin or EDTA blocked adhesion of U937 cells to thrombin-activated endothelia by about 90%. Overall, the pattern of inhibition of P-selectin–dependent cell-cell adhesion by the various heparinoids was similar to that described in the selectin-ligand and cell-selectin assays (Figures 2 and 3). OS-heparin and 2/3DS-heparin were much more potent than the other heparinoids, giving IC50 values within a factor of 5 of native heparin (Table 1). Again, 6DS-heparin was least effective (IC50 ∼350 μg/ml).
Figure 4.
Inhibition of U937 cell adhesion to activated human lung microvascular endothelial cells by modified heparins. Inclusion of a mAb to P-selectin or EDTA blocked binding by more than 90%. Each point represents the average of duplicate determinations, and the data are representative of three or four separate experiments.
Structural analysis of 6DS-heparin.
As the three assay systems demonstrated a critical role for the 6-O-sulfate group in the interaction with L- and P-selectin, detailed analytical studies of this preparation were done. 13C-NMR spectra of native heparin and 6DS-heparin (Figure 5, a and b, respectively) showed characteristic peaks at 69.4 ppm for sulfate esters at C6 of GlcNAc and GlcNSO3 residues and 62.6 ppm for residues unsubstituted at C6. In the heparin spectrum, the relative peak intensities suggested that about 85% of the glucosamine residues were 6-O-sulfated and about 15% were unsulfated. In contrast, 6DS-heparin had no peak at 69.4 ppm, and a dramatic increase in signal at 62.6 ppm. By these criteria, chemical removal of the 6-O-sulfate groups appeared to be nearly quantitative.
Figure 5.
Analysis of heparin and 6DS-heparin. Samples of heparin and 6DS-heparin were analyzed by 13C-NMR (a and b) and nitrous acid degradation to disaccharides (c and d) (see Methods). (a and b) The positions of peaks 1 and 2 correspond to C6 of 6-O-sulfated and unsubstituted glucosamine units, respectively. (c and d) Disaccharides generated by nitrous acid deamination of heparin and 6DS-heparin, respectively. 1, aManR (anhydromannitol), GlcA-aManR, and IdoA-aManR; 2, IdoA-(2OSO3)-aManR; 3, GlcA(2OSO3)-aManR and GlcA-aManR(6OSO3); 4, IdoA-aManR(6OSO3); 5, GlcA-aManR(3OSO3)(6OSO3); 6, IdoA(2OSO3)-aManR(6OSO3); 7, GlcA(2OSO3)-aManR(6OSO3).
To confirm these findings chemically, the disaccharide composition of heparin and 6DS-heparin was determined. The preparations were N-deacetylated by hydrazinolysis and completely depolymerized to disaccharides by treatment with nitrous acid at pH 4 and 1.5. The liberated disaccharides were reduced with NaB[3H]4 and analyzed by reverse-phase ion-pairing chromatography (36), which separates nonsulfated, monosulfated, and disulfated disaccharides with characteristic elution positions (Figure 5, b and d). The major peak (peak 6) represents the fully sulfated disaccharide characteristic of heparin (Figure 1) and was derived from IdoA2OSO3-GlcNAc/SO3(6OSO3) units in the chain. Other 6-O-sulfated disaccharides were derived from IdoA-GlcNAc/SO3(6OSO3) (peak 4), GlcA-GlcNAc/SO3(6OSO3) (peak 5), and GlcA2OSO3-GlcNAc/SO3(6OSO3) (peak 7). Profiles of disaccharides from 6DS-heparin showed complete loss of these 6-O-sulfated units, accompanied by a dramatic increase of nonsulfated disaccharides (peak 1, representing a mixture of disaccharides derived from IdoA-GlcNAc/SO3 and GlcA-GlcNAc/SO3). Based on these data, the extent of 6-O-desulfation was estimated to be greater than 99% with a concomitant loss of about 20% of the 2-O-sulfate groups, as observed previously (34). Since extensive 2-O-desulfation in 2DS-heparin (estimated at >95% by disaccharide analysis) had much less effect on selectin-mediated binding and adhesion events (Figures 2–2), the dramatic decrease in inhibitory activity of 6DS-heparin was primarily due to removal of 6-O-sulfate groups.
Inhibition of thioglycollate-induced acute peritoneal inflammation.
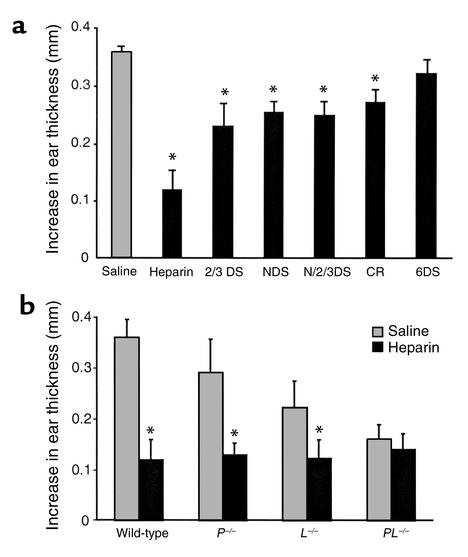
One caveat of the adhesion assays described in Figures 3 and 4 is that cell attachment was performed under static conditions as opposed to conditions of shear flow that would be encountered in the circulation. To examine the physiological relevance of the results, we explored the effects of the heparin derivatives in vivo in two inflammatory models. Thioglycollate injection into the mouse peritoneal cavity induces acute inflammation and neutrophil infiltration that is dependent on both L- and P-selectin (18, 37, 43–45). In control experiments, thioglycollate induced an approximately 120-fold increase of Gr-1–positive neutrophils in the peritoneal cavity after 3 hours compared with the saline-injected animals (1.3 × 106 per mouse versus 1 × 104 per mouse, respectively). To test the inhibitory effects of the various heparin preparations, the compounds were injected intravenously at dosages of 0.5 and 1.25 mg per mouse, 5 minutes after thioglycollate injection. At low dosage, OS-heparin and native heparin inhibited neutrophil recruitment into the peritoneal cavity by 98% and 89%, respectively, compared with saline (Figure 6a; P < 0.001). All of the undersulfated heparins were ineffective at the 0.5 mg dose. When the dosage was increased to 1.25 mg per mouse, 2/3DS-heparin, NDS-heparin, N/2/3DS-heparin, and CR-heparin showed 50–60% inhibition (P < 0.05), but 6DS-heparin lacked activity even at the higher dose (Figure 6a). All of the desulfated preparations and CR-heparin had greatly reduced anticoagulant activity as measured by factor Xa attenuation by antithrombin (Table 1). Thus, the heparinoids could be administered at high dosage to achieve anti-inflammatory effects without unwanted bleeding.
Figure 6.
Inhibition of thioglycollate-induced peritoneal inflammation. (a) Heparin and the various analogs at 0.5 (gray bars) or 1.25 mg per mouse (black bars) were injected intravenously 5 minutes after thioglycollate was injected intraperitoneally. The number of Gr-1–positive granulocytes in the peritoneal cavity was quantitated after 3 hours (see Methods). *Significant difference in neutrophil counts in the control mice that received standard heparin versus those injected with the indicated heparin derivatives. (b) Wild-type C57BL/6 and selectin-deficient mice were injected with heparin (0.5 mg) and thioglycollate. *Significant difference in neutrophil counts in mice treated with heparin (black bars) versus control mice that received only saline (gray bars). Each bar represents the average value ± SD; n = 5–10.
To address whether the inhibition of peritonitis by heparin was L- and P-selectin–dependent, inhibition studies were performed in L-selectin– and P-selectin–deficient mice. Compared with that of wild-type mice, neutrophil recruitment into the peritoneal cavity in L–/– and P–/– mice was reduced by about 50% (Figure 6b). The PL–/– mice also exhibited reduced infiltration, but the level was not lower than observed in each single deficient strain, in contrast to previous findings (37). Possible explanations for the higher background level of leukocyte recruitment in PL–/– mice include (a) the existence of P- and L-selectin–independent pathways that are upregulated due to the simultaneous deletion of both selectins, (b) underlying leukocytosis, and (c) differences in the source of mice: the P–/– and L–/– mice were obtained from The Jackson Laboratory, whereas the PL–/– mice were bred on site and possibly may have some base-line level of chronic inflammation that had upregulated other cell adhesion pathways. Regardless of the reason, the higher level of infiltration makes the lack of effect by injected heparin even more dramatic. Heparin (0.5 mg/mouse) further inhibited the accumulation of neutrophils in both L–/– and P–/– mice but had no effect on infiltration observed in the doubly deficient PL–/– mice. The lack of additive effects in the heparin-treated, doubly deficient mice confirmed that thioglycollate-induced peritonitis was both P- and L-selectin–dependent, and that the anti-inflammatory effect of the heparinoids was mediated primarily by blocking these receptors.
Inhibition of DTH contact dermatitis by heparinoids.
To determine whether the inhibitory effect of heparin in the peritonitis model could be generalized to other forms of inflammation, we examined its effect on acute contact dermatitis, a form of DTH. This model involves sensitization by oxazolone by epicutaneous immunization followed by challenge with topically applied oxazolone to the ear. The reaction is characterized by local accumulation of T lymphocytes, monocytes, and neutrophils, leading to an increase in ear thickness. Treatment of elicited mice with 1 mg heparin reduced ear swelling by 67% (P < 0.005), whereas 6DS-heparin had no obvious inhibitory effect (Figure 7a). The other heparinoids reduced ear swelling to differing extents, but not as dramatically as heparin (25–35%, P < 0.05). Of the modified compounds, 2/3DS-heparin had the highest activity (Figure 7a). Frozen sections of the ear biopsies examined by hematoxylin-eosin staining showed large inflammatory cell infiltrates 24 hours after oxazolone application. The intravenous injection of the heparinoids dramatically reduced the number of both mononuclear and polymorphonuclear leukocytes, which correlated with the decrease in ear swelling. The ears of oxazolone-treated mice injected with saline or 6DS-heparin were similarly edematous and had large numbers of infiltrating leukocytes.
Figure 7.
Inhibition of oxazolone-induced ear swelling in sensitized mice. Mice were sensitized to oxazolone and rechallenged by topical treatment of one ear (see Methods). Swelling was measured by ear thickness, and the value obtained from the vehicle-treated control ear was subtracted from those obtained from oxazolone-treated mice. (a) Animals received a single intravenous injection of heparin (1 mg), heparin derivative (1 mg), or saline within 30 minutes after antigen challenge. *Significant difference in ear swelling in the control mice injected with saline (gray bar) versus those that were injected with the heparin derivatives (black bars). (b) Sensitized wild-type and selectin-deficient mice were injected with heparin (1 mg) and rechallenged with antigen as in a. Each bar represents the average value ± SD; n = 5–8. *Significant difference in ear swelling in mice treated with heparin (black bars) versus control mice that received only saline (gray bars).
Acute contact dermatitis was also induced in P–/–, L–/–, and PL–/– mice. All three selectin-deficient mice exhibited impaired reactivity (20%, 38%, and 56% inhibition, respectively; Figure 7b). Treatment with heparin (1 mg/mouse) further inhibited the reaction in both L–/– and P–/– mice, but not in PL–/– mice. These data confirm that the anti-inflammatory effects of heparin are primarily mediated by blocking P- and L-selectin–dependent reactions.
Discussion
In this report, we have shown that the anti-inflammatory effects of heparin in vivo depend primarily on P- and L-selectins, and that the 6-O-sulfate group of glucosamine units in heparin is critical for interaction with P- and L-selectins. Additionally, the 6-O-sulfate group is critical for both anticoagulant and anti-inflammatory activity, and 2-O,3-O-desulfation of heparin generates a potent, non-anticoagulant, anti-inflammatory agent. These findings suggest that modified heparins may have value as therapeutic agents to block unwanted selectin-dependent reactions.
Much work has been done to find inhibitors to interrupt abnormal leukocyte emigration into tissues during pathological situations (reviewed in refs. 46, 47). SLeX and various analogs have been studied extensively for this purpose (48–56). Other selectin antagonists include mAb’s (57, 58) and SLeX mimetics (49–56, 59, 60), as well as recombinant PSGL-1 (61, 62). As these selectin inhibitors have various drawbacks, such as narrow cross-reactivity, weak affinity, relatively little selectivity among the selectins, short circulating half-life, great expense to produce in the quantities required for treatment, potential antigenicity, and a very limited track record as intravenous therapeutic agents, the development of these compounds into effective drugs for clinical use has been greatly limited. A search for different classes of glycoconjugates that bind to selectins therefore is worthwhile. Several studies have indicated that heparin and heparan sulfate are ligands for L-selectin and P-selectin and will block their binding to SLeX and SLeX-related compounds (17–21, 26, 28, 63). Thus, heparin-related structures might be developed as a therapeutic agent for treating undesirable inflammation.
Binding of heparin to L- and P-selectin depends on both sulfation and molecular size (18, 21), but detailed structural analysis of the binding oligosaccharide sequence has not been done. N-desulfation, O-desulfation at C2/C3, and reduction of carboxyl groups on the uronic acids reduced the ability of heparin to compete for binding and its in vivo anti-inflammatory activities, but the residual activity in these preparations suggests that these groups may not be crucial for binding. In contrast, 6-O-desulfation had a profound effect, indicating an essential role of GlcNAc(6OSO3) or GlcNSO3(6OSO3) residues in binding. In rabbits, microinfusion of heparin, dextran sulfate, and chondroitin-6-sulfate reduced the proportion of rolling cells in isolated venules and increased the number of free-flowing cells (64). Interestingly, the dose range used in this earlier study was comparable to the effective dose of heparin required to block adhesion in vitro (Figures 2–2) and leukocyte infiltration in vivo (Figures 6 and 7). These agents also contain sulfate esters at carbon-6 of glucose, N-acetylglucosamine, or N-acetylgalactosamine, respectively, suggesting a common structural determinant. Previous studies showed that Neu5Acα2,3(6OSO3)Galβ1,4(Fucα1,3)GlcNAcβ- and Neu5Acα2,3Galβ1,4(Fucα1,3) (6OSO3)GlcNAcβ- in GlyCAM-1 and CD34 interacted with P- and L-selectin in high endothelial venules with higher affinity than did their nonsulfated counterparts (65–67). Thus, the 6-O-sulfated glucosamine residues in heparin may occupy the same site in the selectins that binds the sulfated GlcNAc units in these ligands.
Somewhat unexpectedly, heparin blocked ear swelling in P–/– mice and diminished swelling in non–heparin-treated L–/– mice. Catalina et al. reported that the effector phase of contact skin hypersensitivity (CSH) is independent of L-selectin, based on the inability of anti–L-selectin antibodies or L-selectin deficiency to block ear swelling (68, 69). The only L-selectin–dependent step in CSH was infiltration of antigen-specific T cells into the draining nodes after antigen challenge. In the studies reported here, a decrease in ear swelling was observed in the L–/– mice (Figure 7b), which presumably reflects a diminished T cell infiltration into the nodes. The heparin-dependent blockade under these conditions is readily explained by its ability to inhibit P-selectin–dependent infiltration of effector cells (monocytes and neutrophils), since a similar effect was observed using anti–P-selectin blocking antibody in the L–/– mouse (69). In contrast, the ability of heparin to block the CSH response in P–/– mice is not readily reconciled with previous data indicating the independence of the reaction to L-selectin. Perhaps the difference is related to the concentration and volume of sensitizing/rechallenging hapten (oxazolone). Indeed, Xu et al. reported effects more closely related to our findings using significantly larger doses of hapten for elicitation (70). Under these conditions, nonspecific effector cells may have been recruited, and prior studies from a number of groups have indicated that rolling and infiltration under these conditions have an L-selectin–mediated component (37, 71, 72).
As a drug, heparin has wide array of potential pharmacological uses. In the present study, heparin showed strong inhibitory effects in experimental inflammation models. Heparin is known to have inhibitory effects on multiple components of the inflammation cascade, including integrins, cytokines, neutrophil-derived elastases, complement activation, and platelet-activating factor– and TNF-α–induced lung edema (22). However, to our knowledge this is the first report demonstrating that all of the in vivo anti-inflammatory effects of heparin reflect its action on L- and P-selectins. This can be explained by the fact that P- and L-selectin–based interactions are a necessary first step in the inflammatory cascade, occurring before the recruitment of integrins, cytokines, proteases, etc. Heparin could also affect downstream steps in the cascade by inhibiting the interaction of chemokines with lumenally exposed heparan sulfate (73).
In clinical practice, heparin is used as an anticoagulant. For it to be used as an anti-inflammatory drug, the risk of inducing bleeding must be abrogated. Partial chemical desulfation or prefractionation of heparin over antithrombin columns showed that the anti-inflammatory effects of heparin are independent of anticoagulant activity (25, 28, 33, 34). In contrast, removal of sulfate groups from C2 of the uronic acids and, more importantly, from C3 of glucosamine units has a dramatic effect on anticoagulant activity and only slight effects on anti-inflammatory activity properties. However, the processes involved in validating this approach in the clinical setting and getting approval for its general use will likely take a long time. Meanwhile, the currently approved heparin preparations should be investigated for their potential use as anti-inflammatory agents at sub-anticoagulant doses. Indeed, recent data on the therapeutic benefits of low-dose heparin in ulcerative colitis (74), in lichen planus, a form of chronic dermatitis (75), and in ischemic conditions like coronary arterial diseases (76) suggest that this approach may meet with success.
Acknowledgments
This work was supported by grants HL-23594 and HL-57345 from the NIH (to J.D. Esko). We thank Yutaka Kariya for the kind gift of 6-O-desulfated and 2,3-O-desulfated heparin standards, Herman van Halbeek and the Glycotechnology Core at the University of California, San Diego (UCSD), for help in interpreting the NMR spectra, Mark Wahrenbrock (UCSD) for performing endotoxin tests on the heparin samples, Richard O. Hynes for providing PL–/– mice, Lubor Borsig (UCSD) for help in breeding the selectin-deficient mice, Richard Gallo (UCSD) for providing advice on ACD assays, Nissi Varki for histological assays, and Dzung Le and David Ditto (UCSD) for determining anti-Xa activity of the heparinoids.
Footnotes
Conflict of interest: No conflict of interest has been declared.
Nonstandard abbreviations used: sialyl LewisX (SLeX); P-selectin glycoprotein ligand-1 (PSGL-1); glycosylation-dependent cell adhesion molecule-1 (GlyCAM-1); N-desulfated/N-acetylated heparin (NDS-heparin); oversulfated heparin (OS-heparin); carboxyl-reduced heparin (CR-heparin); 6-O-desulfated heparin (6DS-heparin); 2-O,3-O-desulfated heparin (2/3DS-heparin); N-,2-O,3-O-desulfated heparin (N/2/3DS-heparin); polyacrylamide-SLeX (PAA-SLeX); allergic contact dermatitis (ACD); delayed-type hypersensitivity (DTH); contact skin hypersensitivity (CSH).
References
- 1.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 2.McEver RP, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- 3.Lasky LA. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu Rev Biochem. 1995;64:113–139. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- 4.Nelson RM, Venot A, Bevilacqua MP, Linhardt RJ, Stamenkovic I. Carbohydrate-protein interactions in vascular biology. Annu Rev Cell Biol. 1995;11:601–631. doi: 10.1146/annurev.cb.11.110195.003125. [DOI] [PubMed] [Google Scholar]
- 5.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 6.Hynes RO, Wagner DD. Genetic manipulation of vascular adhesion molecules in mice. J Clin Invest. 1996;98:2193–2195. doi: 10.1172/JCI119027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varki A. Selectin ligands: will the real ones please stand up? J Clin Invest. 1997;99:158–162. doi: 10.1172/JCI119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butcher EC, Picker LJ. Lymphocyte homing and homeo-stasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 9.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 10.Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen SD, Bertozzi CR. The selectins and their ligands. Curr Opin Cell Biol. 1994;6:663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 12.Leppanen A, White SP, Helin J, McEver RP, Cummings RD. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J Biol Chem. 2000;275:39569–39578. doi: 10.1074/jbc.M005005200. [DOI] [PubMed] [Google Scholar]
- 13.Maly P, et al. The alpha(1, 3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 14.Becker DJ, Lowe JB. Leukocyte adhesion deficiency type II. Biochim Biophys Acta. 1999; 1455:193–204. doi: 10.1016/s0925-4439(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 15.Homeister JW, et al. The alpha(1, 3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 16.Hayward R, Nossuli TO, Lefer AM. Heparinase III exerts endothelial and cardioprotective effects in feline myocardial ischemia-reperfusion injury. J Pharmacol Exp Ther. 1997;283:1032–1038. [PubMed] [Google Scholar]
- 17.Skinner MP, Lucas CM, Burns GF, Chesterman CN, Berndt MC. GMP-140 binding to neutrophils is inhibited by sulfated glycans. J Biol Chem. 1991;266:5371–5374. [PubMed] [Google Scholar]
- 18.Nelson RM, et al. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82:3253–3258. [PubMed] [Google Scholar]
- 19.Norgard-Sumnicht KE, Varki NM, Varki A. Calcium-dependent heparin-like ligands for L-selectin in nonlymphoid endothelial cells. Science. 1993;261:480–483. doi: 10.1126/science.7687382. [DOI] [PubMed] [Google Scholar]
- 20.Norgard-Sumnicht KE, Varki A. Endothelial heparan sulfate proteoglycans that bind to L-selectin have glucosamine residues with unsubstituted amino groups. J Biol Chem. 1995;270:12012–12024. doi: 10.1074/jbc.270.20.12012. [DOI] [PubMed] [Google Scholar]
- 21.Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest. 1998;101:877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyrrell DJ, Horne AP, Holme KR, Preuss JM, Page CP. Heparin in inflammation: potential therapeutic applications beyond anticoagulation. Adv Pharmacol. 1999;46:151–208. doi: 10.1016/s1054-3589(08)60471-8. [DOI] [PubMed] [Google Scholar]
- 23.Jansen CR, et al. Studies on lymphocytes. II. The production of lyphocytosis by intravenous heparin in calves. Blood. 1962;20:443–451. [PubMed] [Google Scholar]
- 24.Sasaki S. Production of lymphocytosis by polysaccharide polysulphates (heparinoids) Nature. 1967;214:1041–1042. doi: 10.1038/2141041a0. [DOI] [PubMed] [Google Scholar]
- 25.Sy MS, et al. Inhibition of delayed-type hypersensitivity by heparin depleted of anticoagulant activity. Cell Immunol. 1983;82:23–32. doi: 10.1016/0008-8749(83)90137-5. [DOI] [PubMed] [Google Scholar]
- 26.Borsig L, et al. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001;98:3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esko JD, Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest. 2001;108:169–173. doi:10.1172/JCI200113530. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie X, et al. Inhibition of selectin-mediated cell adhesion and prevention of acute inflammation by nonanticoagulant sulfated saccharides: studies with carboxyl-reduced and sulfated heparin and with trestatin A sulfate. J Biol Chem. 2000;275:34818–34825. doi: 10.1074/jbc.M001257200. [DOI] [PubMed] [Google Scholar]
- 29.Nagasawa K, Inoue Y, Kamata T. Solvolytic desulfation of glycosaminoglycuronan sulfates with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1977;58:47–55. doi: 10.1016/s0008-6215(00)83402-3. [DOI] [PubMed] [Google Scholar]
- 30.Höök M, Riesenfeld J, Lindahl U. N-[3H]acetyl-labeling, a convenient method for radiolabeling of glycosaminoglycans. Anal Biochem. 1982;119:236–245. doi: 10.1016/0003-2697(82)90580-2. [DOI] [PubMed] [Google Scholar]
- 31.Wessel HP, Hosang M, Tschopp TB, Weimann BJ. Heparin, carboxyl-reduced sulfated heparin, and Trestatin A sulfate. Antiproliferative and anticoagulant activities. Carbohydr Res. 1990;204:131–139. doi: 10.1016/0008-6215(90)84028-s. [DOI] [PubMed] [Google Scholar]
- 32.Shively JE, Conrad HE. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976;15:3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- 33.Fryer A, et al. Selective O-desulfation produces nonanticoagulant heparin that retains pharmological activity in the lung. J Pharmacol Exp Ther. 1997;282:208–219. [PubMed] [Google Scholar]
- 34.Kariya Y, et al. Preparation of completely 6-O-desulfated heparin and its ability to enhance activity of basic fibroblast growth factor. J Biol Chem. 2000;275:25949–25958. doi: 10.1074/jbc.M004140200. [DOI] [PubMed] [Google Scholar]
- 35.Buchanan MR, Boneu B, Ofosu F, Hirsh J. The relative importance of thrombin inhibition and factor Xa inhibition to the antithrombotic effects of heparin. Blood. 1985;65:198–201. [PubMed] [Google Scholar]
- 36.Guo YC, Conrad HE. Analysis of oligosaccharides from heparin by reversed-phase ion-pairing high-performance liquid chromatography. Anal Biochem. 1988;168:54–62. doi: 10.1016/0003-2697(88)90009-7. [DOI] [PubMed] [Google Scholar]
- 37.Robinson SD, et al. Multiple, targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment. Proc Natl Acad Sci USA. 1999;96:11452–11457. doi: 10.1073/pnas.96.20.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar AK, Rostand KS, Jain RK, Matta KL, Esko JD. Fucosylation of disaccharide precursors of sialyl LewisXinhibit selectin-mediated cell adhesion. J Biol Chem. 1997;272:25608–25616. doi: 10.1074/jbc.272.41.25608. [DOI] [PubMed] [Google Scholar]
- 39.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 40.Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 41.Ramos CL, et al. Functional characterization of L-selectin ligands on human neutrophils and leukemia cell lines: evidence for mucinlike ligand activity distinct from P-selectin glycoprotein ligand-1. Blood. 1998;91:1067–1075. [PubMed] [Google Scholar]
- 42.Collins PW, Macey MG, Cahill MR, Newland AC. von Willebrand factor release and P-selectin expression is stimulated by thrombin and trypsin but not IL-1 in cultured human endothelial cells. Thromb Haemost. 1993;70:346–350. [PubMed] [Google Scholar]
- 43.Mulligan MS, et al. Protective effects of oligosaccharides in P-selectin-dependent lung injury. Nature. 1993;364:149–151. doi: 10.1038/364149a0. [DOI] [PubMed] [Google Scholar]
- 44.Tedder TF, Steeber DA, Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 46.Lowe JB, Ward PA. Therapeutic inhibition of carbohydrate-protein interactions in vivo. J Clin Invest. 1997;99:822–826. doi: 10.1172/JCI119244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefer DJ. Pharmacology of selectin inhibitors in ischemia/reperfusion states. Annu Rev Pharmacol Toxicol. 2000;40:283–294. doi: 10.1146/annurev.pharmtox.40.1.283. [DOI] [PubMed] [Google Scholar]
- 48.Takada A, et al. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53:354–361. [PubMed] [Google Scholar]
- 49.Narasinga Rao BN, et al. Sialyl Lewis X mimics derived from a pharmacophore search are selectin inhibitors with anti-inflammatory activity. J Biol Chem. 1994;269:19663–19666. [PubMed] [Google Scholar]
- 50.Buerke M, et al. Sialyl LewisX-containing oligosaccharide attenuates myocardial reperfusion injury in cats. J Clin Invest. 1994;93:1140–1148. doi: 10.1172/JCI117066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lefer DJ, Flynn DM, Phillips ML, Ratcliffe M, Buda AJ. A novel sialyl Lewisxanalog attenuates neutrophil accumulation and myocardial necrosis after ischemia and reperfusion. Circulation. 1994;90:2390–2401. doi: 10.1161/01.cir.90.5.2390. [DOI] [PubMed] [Google Scholar]
- 52.Maaheimo H, Renkonen R, Turunen JP, Penttila L, Renkonen O. Synthesis of a divalent sialyl Lewis x O-glycan, a potent inhibitor of lymphocyte-endothelium adhesion. Evidence that multivalency enhances the saccharide binding to L-selectin. Eur J Biochem. 1995;234:616–625. doi: 10.1111/j.1432-1033.1995.616_b.x. [DOI] [PubMed] [Google Scholar]
- 53.DeFrees SA, Phillips L, Guo L, Zalipsky S. Sialyl Lewis x liposomes as a multivalent ligand and inhibitor of E-selectin mediated cellular adhesion. J Am Chem Soc. 1996;118:6101–6104. [Google Scholar]
- 54.Nguyen M, Eilber FR, DeFrees S. Novel synthetic analogs of sialyl Lewis X can inhibit angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 1996;228:716–723. doi: 10.1006/bbrc.1996.1722. [DOI] [PubMed] [Google Scholar]
- 55.Renkonen O, et al. Synthesis of a new nanomolar saccharide inhibitor of lymphocyte adhesion: different polylactosamine backbones present multiple sialyl Lewis x determinants to L-selectin in high-affinity mode. Glycobiology. 1997;7:453–461. doi: 10.1093/glycob/7.4.453-c. [DOI] [PubMed] [Google Scholar]
- 56.Sanders WJ, Manning DD, Koeller KM, Kiessling LL. Synthesis of sulfated trisaccharide ligands for the selectins. Tetrahedron. 1997;53:16391–16422. [Google Scholar]
- 57.Chen LY, Nichols WW, Hendricks JB, Yang BC, Mehta JL. Monoclonal antibody to P-selectin (PB1.3) protects against myocardial reperfusion injury in the dog. Cardiovasc Res. 1994;28:1414–1422. doi: 10.1093/cvr/28.9.1414. [DOI] [PubMed] [Google Scholar]
- 58.Lefer DJ, Flynn DM, Buda AJ. Effects of a monoclonal antibody directed against P-selectin after myocardial ischemia and reperfusion. Am J Physiol. 1996;270:H88–H98. doi: 10.1152/ajpheart.1996.270.1.H88. [DOI] [PubMed] [Google Scholar]
- 59.Skurk C, Buerke M, Guo J-P, Paulson J, Lefer AM. Sialyl Lewisx-containing oligosaccharide exerts beneficial effects in murine traumatic shock. Am J Physiol Heart Circ Physiol. 1994;267:H2124–H2131. doi: 10.1152/ajpheart.1994.267.6.H2124. [DOI] [PubMed] [Google Scholar]
- 60.Sanders WJ, et al. Inhibition of L-selectin-mediated leukocyte rolling by synthetic glycoprotein mimics. J Biol Chem. 1999;274:5271–5278. doi: 10.1074/jbc.274.9.5271. [DOI] [PubMed] [Google Scholar]
- 61.Borges E, et al. The P-selectin glycoprotein ligand-1 is important for recruitment of neutrophils into inflamed mouse peritoneum. Blood. 1997;90:1934–1942. [PubMed] [Google Scholar]
- 62.Hayward R, Campbell B, Shin YK, Scalia R, Lefer AM. Recombinant soluble P-selectin glycoprotein ligand-1 protects against myocardial ischemic reperfusion injury in cats. Cardiovasc Res. 1999;41:65–76. doi: 10.1016/s0008-6363(98)00266-1. [DOI] [PubMed] [Google Scholar]
- 63.Zakrzewicz A, et al. L-selectin-dependent leukocyte adhesion to microvascular but not to macrovascular endothelial cells of the human coronary system. Blood. 1997;89:3228–3235. [PubMed] [Google Scholar]
- 64.Ley K, Cerrito M, Arfors KE. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol. 1991;260:1667–1673. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- 65.Hemmerich S, Bertozzi CR, Leffler H, Rosen SD. Identification of the sulfated monosaccharides of GlyCAM-1, an endothelial derived ligand for L-selectin. Biochemistry. 1994;33:4820–4829. doi: 10.1021/bi00182a010. [DOI] [PubMed] [Google Scholar]
- 66.Hemmerich S, Butcher EC, Rosen SD. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79, an adhesion-blocking monoclonal antibody. J Exp Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hemmerich S, Leffler H, Rosen SD. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J Biol Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- 68.Catalina MD, et al. The route of antigen entry determines the requirement for L-selectin during immune responses. J Exp Med. 1996;184:2341–2351. doi: 10.1084/jem.184.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Catalina MD, Estess P, Siegelman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999;93:580–589. [PubMed] [Google Scholar]
- 70.Xu JC, Grewal IS, Geba GP, Flavell RA. Impaired primary T cell responses in L-selectin-deficient mice. J Exp Med. 1996;183:589–598. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frenette PS, Wagner DD. Insights into selectin function from knockout mice. Thromb Haemost. 1997;78:60–64. [PubMed] [Google Scholar]
- 72.Forlow SB, Ley K. Selectin-independent leukocyte rolling and adhesion in mice deficient in E-, P-, and L-selectin and ICAM-1. Am J Physiol Heart Circ Physiol. 2001;280:H634–H641. doi: 10.1152/ajpheart.2001.280.2.H634. [DOI] [PubMed] [Google Scholar]
- 73.Kuschert GSV, et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 74.Dotan I, et al. Low-dose low-molecular weight heparin (enoxaparin) is effective as adjuvant treatment in active ulcerative colitis. An open trial. Dig Dis Sci. 2001;46:2239–2244. doi: 10.1023/a:1011979418914. [DOI] [PubMed] [Google Scholar]
- 75.Hodak E, et al. Low-dose low-molecular-weight heparin (enoxaparin) is beneficial in lichen planus: a preliminary report. J Am Acad Dermatol. 1998;38:564–568. doi: 10.1016/s0190-9622(98)70118-5. [DOI] [PubMed] [Google Scholar]
- 76.Menon V, Berkowitz SD, Antman EM, Fuchs RM, Hochman JS. New heparin dosing recommendations for patients with acute coronary syndromes. Am J Med. 2001;110:641–650. doi: 10.1016/s0002-9343(01)00715-x. [DOI] [PubMed] [Google Scholar]