Abstract
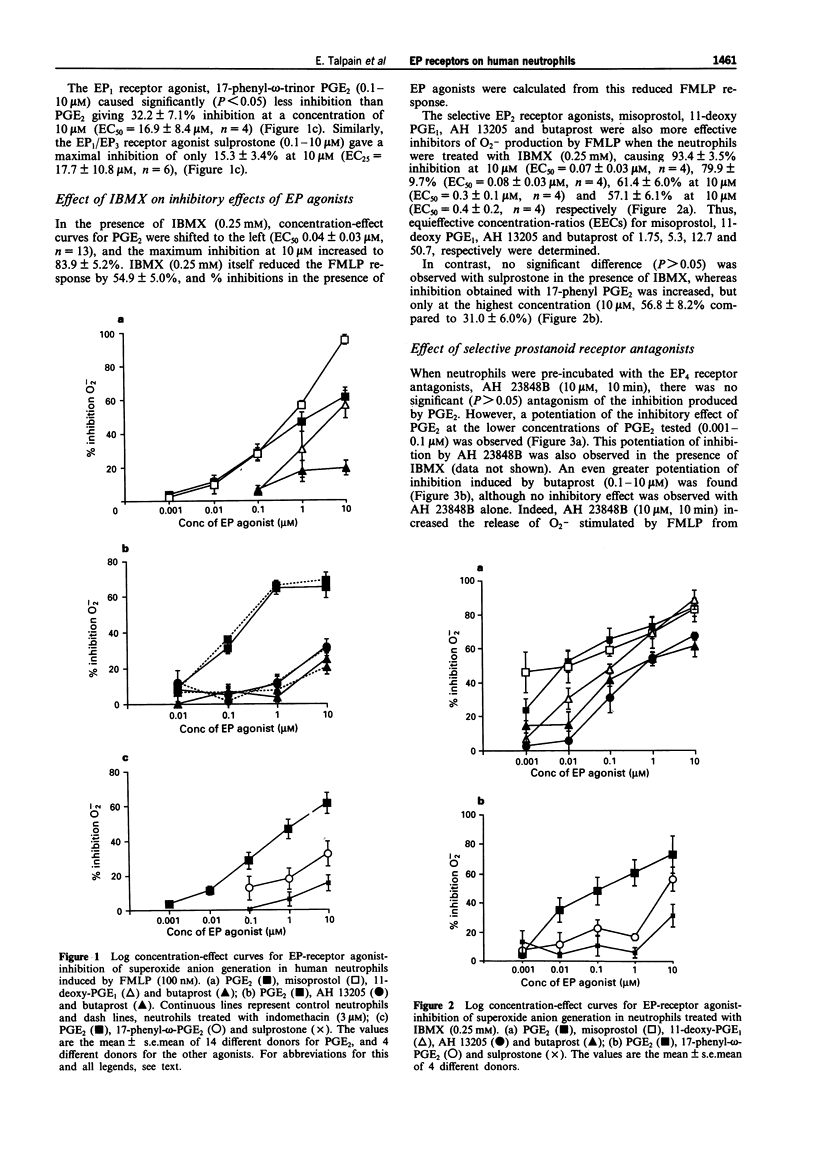
1. The aims of this study were to characterize the EP receptor subtype mediating the inhibition of superoxide anion generation by formyl methionyl leucine phenylalanine (FMLP)-stimulated human neutrophils, and to test the hypothesis that adenosine 3':5'-cyclic monophosphate (cyclic AMP) is the second messenger mediating the inhibition of the neutrophil by prostaglandin (PG)E2. 2. PGE2 (0.001-10 microM) inhibited FMLP (100 nM)-induced O2-generation from human peripheral blood neutrophils in a concentration-dependent manner, with an EC50 of 0.15 +/- 0.03 microM, and a maximum effect ranging from 36-84% (mean inhibition of 68.7 +/- 2.5%, n = 32). 3. The EP2-receptor agonists, misoprostol, 11-deoxy PGE1, AH13205 and butaprost, all at 10 microM, inhibited O2- generation, causing 95.5 +/- 2.9%, 56.8 +/- 5.2%, 37.1 +/- 6.6% and 18.9 +/- 4.4% inhibition respectively, the latter two being much less effective than PGE2. Similarly, the EP1-receptor agonist, 17-phenyl PGE2 (10 microM), and the EP3/EP1-receptor agonist, sulprostone (10 microM), also inhibited O2- generation, causing 32.2 +/- 7.0% and 15.3 +/- 3.4% inhibition respectively. 4. The non-selective phosphodiesterase inhibitor, isobutyl methylxanthine (IBMX, 0.25 mM) inhibited the FMLP response by 54.5 +/- 5.0%. In addition, IBMX shifted concentration-effect curves for PGE2, misoprostol, 11-deoxy PGE1, butaprost, and AH 13205 to the left, to give EC50s of 0.04 +/- 0.03 (n = 13), 0.07 +/- 0.03 (n = 4), 0.08 +/- 0.03 (n = 4), 0.33 +/- 0.13 (n = 4) and 0.41 +/- 0.2 microM (n = 3) respectively, allowing equieffective concentration-ratios (EECs, PGE2 = 1) of 11.5, 5.3, 50.7 and 12.7 to be calculated.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

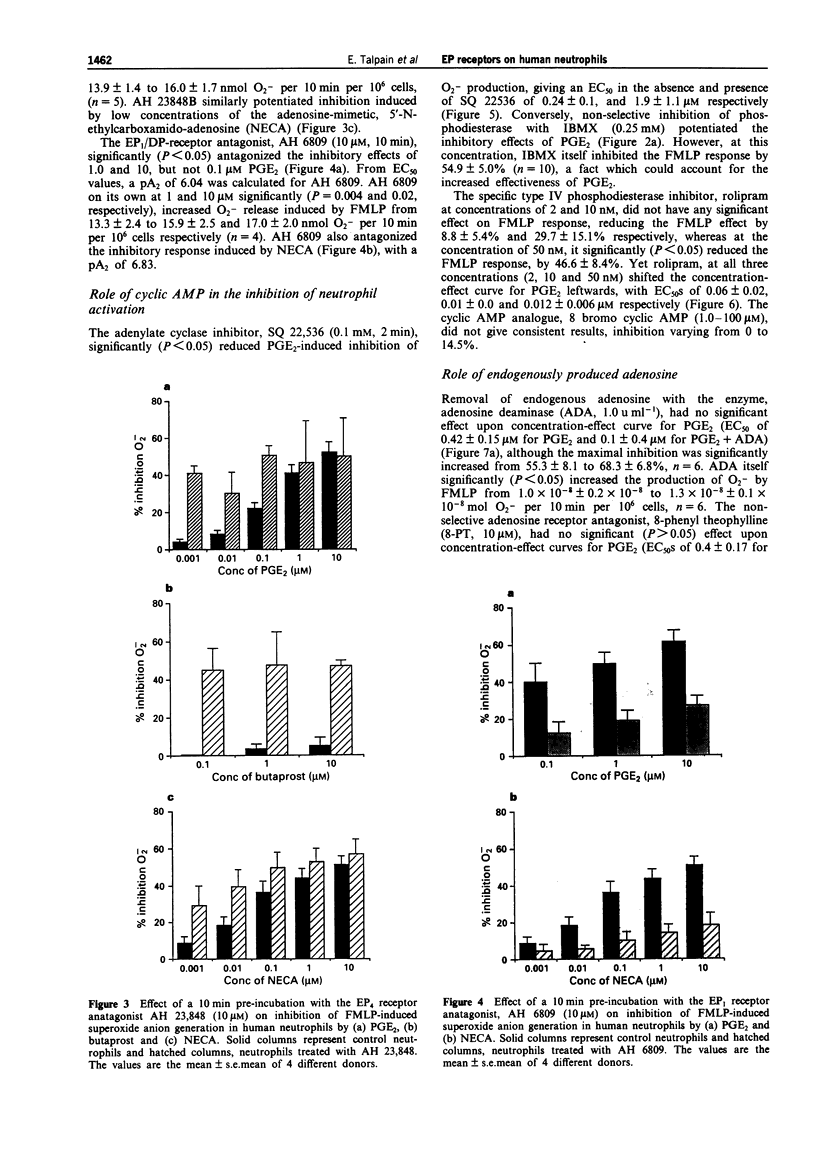
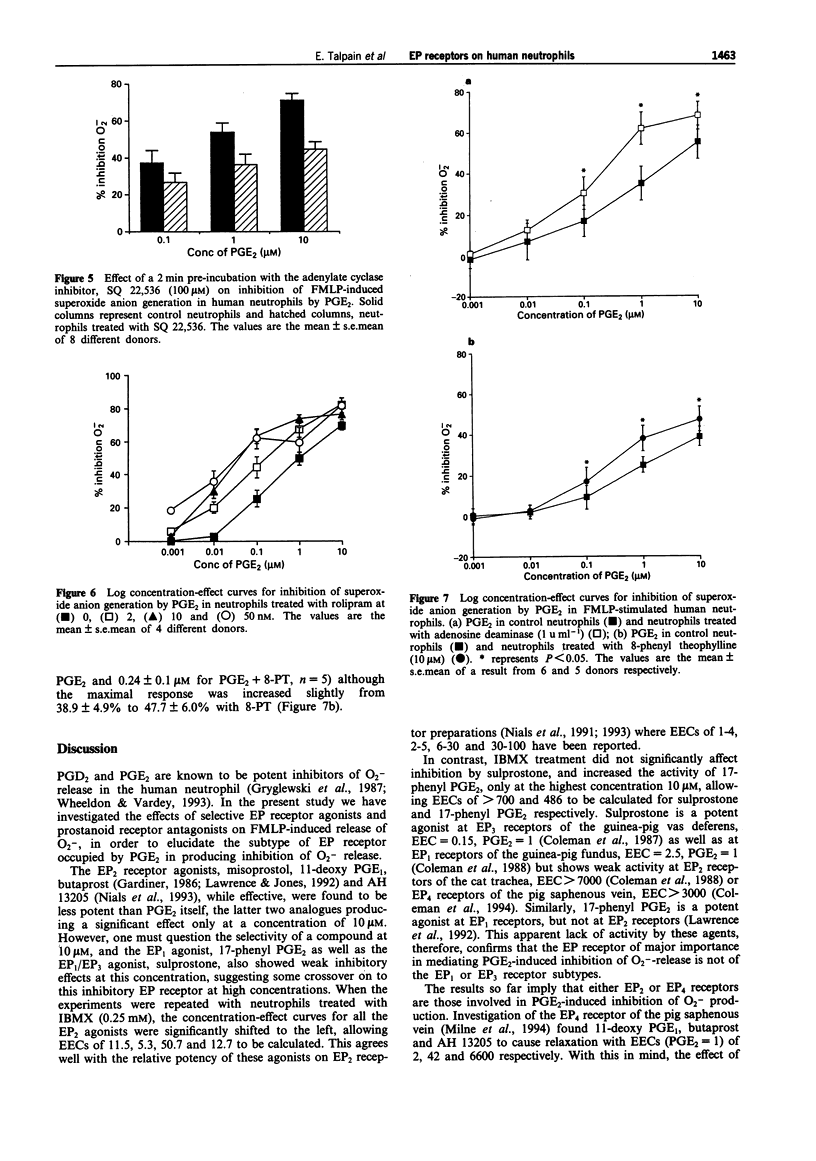


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. A., Talpain E. Comparison of the prostaglandin E (EP) receptor of human neutrophils and HL-60 cells differentiated with DMSO. Prostaglandins. 1994 Oct;48(4):221–234. doi: 10.1016/0090-6980(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lehrer R. I., Lichtenstein L. M., Weissmann G., Zurier R. Effects of cholera enterotoxin on adenosine 3',5'-monophosphate and neutrophil function. Comparison with other compounds which stimulate leukocyte adenyl cyclase. J Clin Invest. 1973 Mar;52(3):698–708. doi: 10.1172/JCI107231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. A., Grix S. P., Head S. A., Louttit J. B., Mallett A., Sheldrick R. L. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins. 1994 Feb;47(2):151–168. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Kennedy I., Sheldrick R. L. New evidence with selective agonists and antagonists for the subclassification of PGE2-sensitive (EP) receptors. Adv Prostaglandin Thromboxane Leukot Res. 1987;17A:467–470. [PubMed] [Google Scholar]
- Collis M. G., Hourani S. M. Adenosine receptor subtypes. Trends Pharmacol Sci. 1993 Oct;14(10):360–366. doi: 10.1016/0165-6147(93)90094-z. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Rosenstein E. D., Korchak H. M., Weissmann G., Hirschhorn R. Occupancy of adenosine receptors raises cyclic AMP alone and in synergy with occupancy of chemoattractant receptors and inhibits membrane depolarization. Biochem J. 1988 Jun 15;252(3):709–715. doi: 10.1042/bj2520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine deaminase is not required for the generation of superoxide anion. Clin Immunol Immunopathol. 1984 Mar;30(3):495–499. doi: 10.1016/0090-1229(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Gardiner P. J. Characterization of prostanoid relaxant/inhibitory receptors (psi) using a highly selective agonist, TR4979. Br J Pharmacol. 1986 Jan;87(1):45–56. doi: 10.1111/j.1476-5381.1986.tb10155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Szczeklik A., Wandzilak M. The effect of six prostaglandins, prostacyclin and iloprost on generation of superoxide anions by human polymorphonuclear leukocytes stimulated by zymosan or formyl-methionyl-leucyl-phenylalanine. Biochem Pharmacol. 1987 Dec 15;36(24):4209–4213. doi: 10.1016/0006-2952(87)90660-5. [DOI] [PubMed] [Google Scholar]
- Harris D. N., Asaad M. M., Phillips M. B., Goldenberg H. J., Antonaccio M. J. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J Cyclic Nucleotide Res. 1979;5(2):125–134. [PubMed] [Google Scholar]
- Hecker G., Ney P., Schrör K. Cytotoxic enzyme release and oxygen centered radical formation in human neutrophils are selectively inhibited by E-type prostaglandins but not by PGI2. Naunyn Schmiedebergs Arch Pharmacol. 1990 Apr;341(4):308–315. doi: 10.1007/BF00180656. [DOI] [PubMed] [Google Scholar]
- Keery R. J., Lumley P. AH6809, a prostaglandin DP-receptor blocking drug on human platelets. Br J Pharmacol. 1988 Jul;94(3):745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. M., Goldberg B. J., Smiley P. A., Olson C. V. Receptor-specific threshold effects of cyclic AMP are involved in the regulation of enzyme release and superoxide production from human neutrophils. Biochim Biophys Acta. 1985 Aug 30;846(2):286–295. doi: 10.1016/0167-4889(85)90076-x. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Jones R. L. Investigation of the prostaglandin E (EP-) receptor subtype mediating relaxation of the rabbit jugular vein. Br J Pharmacol. 1992 Apr;105(4):817–824. doi: 10.1111/j.1476-5381.1992.tb09063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R. A., Jones R. L., Wilson N. H. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br J Pharmacol. 1992 Feb;105(2):271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney P., Schrör K. PGD2 and its mimetic ZK 110.841 are potent inhibitors of receptor-mediated activation of human neutrophils. Eicosanoids. 1991;4(1):21–28. [PubMed] [Google Scholar]
- Schudt C., Winder S., Forderkunz S., Hatzelmann A., Ullrich V. Influence of selective phosphodiesterase inhibitors on human neutrophil functions and levels of cAMP and Cai. Naunyn Schmiedebergs Arch Pharmacol. 1991 Dec;344(6):682–690. doi: 10.1007/BF00174752. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. B., Berube M. L., Zurier R. B. Stimulus-dependent inhibition of superoxide generation by prostaglandins. Clin Immunol Immunopathol. 1985 Feb;34(2):205–215. doi: 10.1016/0090-1229(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Wheeldon A., Vardey C. J. Characterization of the inhibitory prostanoid receptors on human neutrophils. Br J Pharmacol. 1993 Apr;108(4):1051–1054. doi: 10.1111/j.1476-5381.1993.tb13504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K., Freund K. Inhibition of the n-formylmethionyl-leucyl-phenylalanine induced respiratory burst in human neutrophils by adrenergic agonists and prostaglandins of the E series. Can J Physiol Pharmacol. 1981 Sep;59(9):915–920. doi: 10.1139/y81-141. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]


