Abstract
Systemic administration of antigen/peptide for peripheral T cell tolerance has long been investigated as a potential approach to therapy of autoimmune diseases. The multiple antimyelin T cell reactivities likely to be associated with multiple sclerosis (MS) impose major difficulties in devising such an immune-specific therapeutic approach to the disease, because targeting T cells specific for a single autoantigen/epitope is unlikely to be sufficiently effective. Here, we present a pilot study on the possibility of concomitantly inhibiting multiple potentially pathogenic antimyelin T cell reactivities by tolerogenic administration of an artificial “multiantigen/multiepitope” protein. A synthetic gene was constructed to encode selected disease-relevant epitopes of myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG). The protein product, hmTAP (synthetic human multitarget autoantigen protein), was adequately processed for antigenic presentation of the relevant integral epitopes, in vitro and in vivo. Systemic administration of hmTAP not only suppressed and treated experimental autoimmune encephalomyelitis (EAE) initiated by autoreactivity to a PLP epitope, but also abrogated complex EAE transferred by multispecific line T cells reactive against encephalitogenic epitopes of MBP, PLP, and MOG. These data indicate that multiantigen/multiepitope–directed therapy of complex autoimmune diseases is effective and can be mediated by the protein product of a specifically designed synthetic gene.
Introduction
Multiple sclerosis (MS), an inflammatory disease of the CNS characterized by neurological impairment of varying extent, results from demyelination, which is believed to ensue from an autoimmune response against myelin. A number of CNS myelin proteins have been postulated to be potential primary target antigens in MS on the basis of their ability to induce experimental autoimmune encephalomyelitis (EAE) and detection of autoreactivity to these antigens in MS patients (reviewed in refs. 1, 2; 3–7). Among these, myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG) have been extensively studied (reviewed in refs. 1, 2). In addition to primary pathogenic autoreactivities implicated in disease initiation, neo-autoreactivities can emerge as a result of intra- or intermolecular spread of autoimmunity during the course of chronic EAE (8–13) and, as recently suggested, may also be associated with disease progression in MS (13, 14). Such a potential multiplicity of antimyelin autoreactivities imposes major difficulties in devising antigen-specific therapeutic agents for MS, not only in view of the possibility that the primary target antigen and/or emerging neo-reactivities may differ in different patients, but also because potentially pathogenic autoreactivities to several myelin antigens may occur in any one patient at any one time.
Several studies in EAE strongly suggest that neutralizing T cells specific for one epitope may not be a sufficiently effective therapeutic approach for disease associated with multiple pathogenic autoreactivities. Thus, in chronic EAE induced in SJL/J mice with whole PLP, tolerization with the major encephalitogenic peptide, PLP139-151, abrogated the primary acute phase, but not subsequent relapses related to autoimmune spread (11). The clinical severity of EAE induced in (PL/J × SJL/J) F1 mice with a combination of MBP and MOG could be significantly reduced by tolerogenic administration of a combination of the immunodominant encephalitogenic epitopes within MBP Ac1-11 and MOG41-60 (15). In contrast, MBPAc1-11, which suppresses MBP-induced EAE in these F1 mice, had no effect on MOG-induced EAE and a marginal therapeutic effect on EAE induced by the MBP/MOG combination (15), an observation most likely related to its specific suppressive effect on MBP-reactive T cells. Highly relevant to treatment of disease with multiple autoreactivities is the strong therapeutic effect on EAE of MP4, a chimeric fusion protein, comprising the whole long isoform of MBP (21.5 kDa MBP) and the hydrophilic domains of PLP (ΔPLP) (16). Tolerogenic administration of MP4 fully abrogated EAE actively induced with PLP139-151, as well as EAE adoptively transferred with a combination of encephalitogenic MBP- and PLP-specific T cells in SJL/J mice. In contrast, neither 21.5 kDa MBP nor ΔPLP injected individually had any such dramatic effect on passive EAE mediated by the combined T cell populations (16). Taken together, these studies suggest that targeting the majority of relevant T cells may be required for optimal efficacy of immune-specific therapy in disease associated with pathogenic T cell reactivities against more than one antigen/epitope. Here, we present a pilot study of a multitargeting approach to specific immunomodulation of complex EAE associated with multiple pathogenic autoreactivities. A synthetic gene, designated synthetic human multi-target autoantigen gene related to EAE (shMultiTAG/E), was engineered to encode only encephalitogenic and disease-relevant epitopes of MBP, PLP, and MOG. The immunomodulatory effect of its protein product, human multitarget autoantigen protein (hmTAP), was tested on active and passive EAE induced by T cell responses to defined epitopes as well as on complex EAE passively transferred with multireactive T cells.
Methods
Mice.
Female C3H.SW and SJL/J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA) or obtained from the Weizmann Institute of Science colony; (C3H.SW × SJL/J)F1 mice were bred at the Weizmann Institute of Science Animal Facility. All mice were 2–3 months old when used in the experiments.
Synthetic oligonucleotides related to generation, amplification, and cloning of shTAG DNA coding for MOG, MBP, or PLP epitopes.
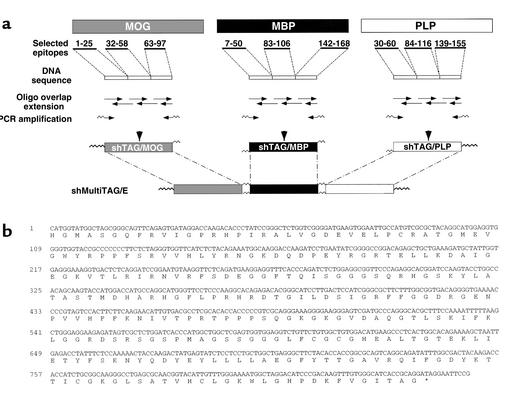
For each shTAG, 60- to 70-nucleotide-long oligonucleotides, which represent codons of the amino acid residues of the selected epitopes aligned sequentially and which are complementary at their 5′ and/or 3′ ends to their neighboring oligonucleotides by an overlap of 15–18 nucleotides, were synthesized by the Weizmann Institute of Science Synthesis Unit. Relevant oligonucleotides include specific restriction endonuclease sites to enable cloning or in-frame ligation to neighboring shTAGs.
Synthetic oligonucleotides used for generation and amplification of shTAG/MOG DNA are as follows:shMOG Ia: 5′-CATGGTATGGCTAGCGGGCAGTTCAGAGTGATAGGACCAAGACACCCT ATCCGGGCTCTGGTC-3′; shMOG Ib: 5′-CATGGTATGGCTAGCGGGCAGTTCAGAGTGATAGGA-3′; shMOG II: (reverse) 5′-CTCCATGCCTGTAGCGCGACATGGCAATTCCACTTCATCCCCG ACCAGAGCCCGGATAGG-3′; shMOG III: 5′-CGCGCTACAGGCATGGAGGTGGGGTGGTACCGCCCCCCCTTCTCTAGG GTGGTTCATCTCTACAGAAAT-3′; shMOG IV: (reverse) 5′-TTTCAGCAGCTCTGTCCGGCCCCGATATTCAGGATCTTG GTCCTTGCCATTTCTGTAGAGATGAAC-3′; shMOG V: 5′-CGGACAGAGCTGCTGAAAGATGCTATTGGTGAGGGAAAGGTGACTCTCA GGATCCGGAATGTAAGG-3′; shMOG VIa: (reverse) 5′-CAGAGAGATCTAGGTGAAACCTCCTTCATCTGAGAACCTTACAT TCCGGATCCT-3′; shMOG VIb: (reverse) 5′-CAGAGAGATCTGGGTGAAACC-3′. The NheI site in shMOG Ia and Ib and the BglII site in shMOG VIa and VIb are underlined.
Synthetic oligonucleotides for generation and amplification of shTAG/MBP DNA are as follows: shMBP Ia: 5′-GAAGATCTCTGGAGGCGGTTCCCAGAGGCACGGATCCAAG-3′; shMBP Ib: 5′-GAAGATCTCTGGAGGCGGT-3′; shMBP Ic: 5′-TTGGACGCTAGCTCCCAGAGGCACGGATCCAAGTACCTG-3′; shMBP II: (reverse) 5′-GAGGAACCCATGCCTGGCATGGTCCATGGTACTTGCTGTGGCCAGGTACTTGGATCCGTGCCTCTG-3′; shMBP III: 5′-GCCAGGCATGGCTTCCTCCCAAGGCACAGAGACACGGGCATCCTTGACTCCATCGGGCGCTTCTTTGGC-3′; shMBP IV: (reverse) 5′-AATGTTCTTGAAGAAGTGGACTACGGGGTTTTCACCCCTGTCAC CGCCAAAGAAGCGCCCGAT-3′; shMBP V: 5′-CACTTCTTCAAGAACATTGTGACGCCTCGCACACCACCCCCGTCGCAGG GAAAGGGG-3′; shMBP VI: 5′-AATTTTGGAAAGCGTGCCCTGGGCATCGACTCCCTTCCCCTTTCCCTGC GACGG-3′; shMBP VII: (reverse) 5′-GGCACGCTTTCCAAAATTTTTAAGCTGGGAGGAAGAGATAGTC GCTCT-3′; shMBP VIIIa: (reverse) 5′-ACCACTCGAGCCAGCCATGGGTGATCCAGAGCGACTATCTCTTC CTCC-3′; shMBP VIIIb (reverse) 5′-ACCACTCGAGCCAGCCATGGGTGATCCAGA-3′; shMBP VIIIc: (reverse) 5′-ACCACTCGAGCTAAGCCATGGGTGATCCAGA-3′. The BglII site in shMBP Ia and Ib, the NheI site in shMBP Ic, and the XhoI site in shMBP VIIIa, VIIIb, and VIIIc are underlined.
Synthetic oligonucleotides for generation and amplification of shTAG/PLP DNA are as follows: shPLP Ia: 5′-AGGCTCGAGTGGTGGAGGTCTGTTCTGTGGCTGTGGACATGAAGCCCTC ACTGGCACAGAAAAGCTA-3′; shPLP Ib: 5′-AGGCTCGAGTGGTGGAGGTCTAGGTCT G-3′; shPLP II: (reverse)5′-GAGGAGATACTCATAGTCTTGGTAGTTTTTGGAGAAATAGGTCT CAATTAGCTTTTCTGTGCCAGT-3′ shPLP III: 5′-GACTATGAGTATCTCCTCCTGCTGGCTGAGGGCTTCTACACCACCGGCGC AGTCAGGCAGATA-3′; shPLP IV: (reverse) 5′-TACCGTTGCGCTCAGGCCCTTGCCGCAGATGGTGGTCTTGTAGT CGCCAAATATCTGCCTGACTGCGCC-3′; shPLP V: 5′-GGCCTGAGCGCAACGGTACATTGTTTGGGAAAATGGCTAGGACATCCCG ACAAGTTTGTGGGCATCACC-3′; shPLP VIa: (reverse) 5′-CGGAATTCCTATCCTGCGGTGATGCCCACAAACTTGTC-3′. The XhoI site in shPLPIa and Ib and the EcoRI site in shPLP VIa are underlined.
Construction, amplification, and cloning of shTAG/MOG, shTAG/MBP, and shTAG/PLP and expression and purification of the protein products.
For the preparation of each shTAG DNA template, the relevant oligonucleotides were mixed (each 75 pmol) in Taq DNA polymerase buffer (40 μl final volume) containing dNTPs (RO181; MBI Fermentas AB, Vilnius, Lithuania) at a final concentration of 0.2 mM each and a mixture of 0.2 U Vent DNA polymerase (2545; New England Biolabs Inc., Beverly, Massachusetts, USA) and 0.5 U Taq DNA polymerase (AB-0192; Advanced Biotechnologies, Surrey, United Kingdom). After denaturation (94°C, 1 minute) and annealing (55°C, 2 minute), PCR overlap extension was carried out (72°C, 5 minute), and the resulting template (4 μl) was PCR amplified (standard conditions, 30 cycles) using the relevant oligonucleotides as primers. The shTAG/MOG DNA template was generated using oligonucleotides shMOG Ia, II, III, IV, V, and VIa and PCR amplified with oligonucleotides shMOG Ib and VIa as forward and reverse primers, respectively. The shTAG/MBP DNA template was generated using oligonucleotides shMBM Ia, II, III, IV, V, VI, VII, and VIIIa and PCR amplified with oligonucleotides shMBP Ib and VIIIb as forward and reverse primers, respectively. The shTAG/PLP DNA template was generated using oligonucleotides shPLP Ia, II, III, IV, V, and VIa and PCR amplified with oligonucleotides shPLP Ib and VIa as forward and reverse primers, respectively. The amplified PCR product of the expected size eluted from agarose gel was directly cloned into a T vector (pGEM-T, A3600; Promega Corp., Madison, Wisconsin, USA) and sequenced using SP6 and T7 specific primers.
The shTAG/MOG DNA excised from pGEMT/shTAG/MOG was cloned into the bacterial expression vector pRSET (V351-20; Invitrogen Corp., San Diego, California, USA) via NheI and BglII, 3′ to its 6xHis tag. The shTAG/MBP DNA was PCR amplified from pGEMT/shTAG/MBP using oligonucleotides shMBP Ic and VIIIc, which introduce an NheI site and a stop codon, respectively, and cloned into pRSET via NheI and XhoI, 3′ to its 6xHis tag. The shTAG/PLP DNA excised from pGEMT/shTAG/PLP was cloned into pRSET via XhoI and EcoRI, 3′ to its 6xHis tag. DNA sequence analysis, using pRSET-specific primers, confirmed an open reading frame with the ATG of pRSET.
The pRSET/shTAG/MOG, pRSET/shTAG/MBP, and pRSET/shTAG/PLP were each transformed into Escherichia coli host (BL21-DE3), and protein expression was induced by isopropyl β-D-thiogalactopyranoside (IPTG) (RO392; MBI Fermentas AB). Strong expression from pRSET/shTAG/MOG was observed, and the expressed protein, shMOG/E, was isolated under denaturing conditions (8 M urea) by metal chelate affinity chromatography on Ni2+ nitriloacetic acid (NTA) agarose (30230; Qiagen Inc., Valencia, California, USA) according to the manufacturer’s protocol. Fractions containing the isolated protein, as evidenced by SDS-PAGE, were pooled and subjected to reducing conditions with β-mercaptoethanol. The protein was diluted to 50–100 μg/ml in 8 M urea and allowed to refold by dialysis against gradually decreasing concentrations of urea (8–0 M). Aggregated protein was removed by centrifugation. Expression of shMBP/E and shPLP/E from pRSET/shTAG/MBP and pRSET/shTAG/PLP, respectively, was not observed.
Construction of shMultiTAG/E and expression of its protein product, hmTAP.
To construct the shMultiTAG/E, shTAG/MBP DNA and shTAG/PLP DNA excised from pGEMT/shTAG/MBP and pGEMT/shTAG/PLP, respectively, were ligated together via XhoI (shTAG/MBP-shTAG/PLP DNA fragment). The shTAG/MOG DNA was PCR amplified from pGEMT/shTAG/MOG using oligonucleotides shMOG Ib and VIb to remove the stop codon. The DNA fragment of the expected size was ligated to the shTAG/MBP-shTAG/PLP DNA fragment via BglII. The resulting shTAG/MOG-shTAG/MBP-shTAG/PLP DNA fragment (shMultiTAG/E) was then cloned into pRSET, via NheI and EcoRI, 3′ to its 6xHis tag. DNA sequence analysis using the pRSET-specific primers confirmed the shMultiTAG/E DNA sequence encoding shTAG/MOG, shTAG/MBP, and shTAG/PLP sequentially as an open reading frame with the ATG of pRSET. Expression of pRSET/shMultiTAG/E in E. coli (BL21-DE3) and purification of its product, hmTAP, was as described above.
Generation of rhMBP and shPLP215.
DNA coding for the 18.5-kDa isoform of human MBP (17) was PCR amplified from the human brain cDNA library (a gift from Orly Reiner, Dept. of Molecular Genetics, The Weizmann Institute of Science) using 5′-CATGGTATGGCTAGCGCGTCACAGAAGAGA-3′ (NheI site underlined) and 5′-AACCAGAAGATCTTAGCGTCTAGCCATGGGTGATCC-3′ (BglII site underlined) as forward and reverse primers, respectively. The resulting DNA fragment was digested and cloned into pRSET via NheI and BamHI, 3′ to its 6xHis tag. DNA sequence analysis using the pRSET primers confirmed the DNA sequence of rhMBP (18.5 kDa MBP) as an open reading frame with the ATG of pRSET. Expression of pRSET/rhMBP into E. coli (BL21-DE3) and purification of rhMBP was as described above.
ShPLP215 corresponds to shPLP/E to which amino acids 215–235 of human PLP were added. It was generated as follows: pGEMT/shTAG/PLP DNA was denatured and annealed with oligonucleotide shPLP215 I (reverse) 5′-GATGGACAGAAGGTTGGAGCCACAAACCTTGCCAGGTGCGGTG ATGCCCACAAACTTGTC-3′ for ten cycles of PCR overlap extension. PCR amplification was continued for 35 cycles using as forward primer oligonucleotide shPLP215 II, 5′-TTGGACGCTAGCCTGTTCTGTGGCTGTGGACAT-3′, rather than shPLP Ib, to introduce an NheI site (underlined). The PCR product of the expected size was annealed to oligonucleotide shPLP215 III (reverse) 5′-GCAAGCTTCTACGCTCCGAACTCAGCTGTTTTGCAGATGGACA GAAGGTTGGAGCC-3′ (HindIII site underlined). After ten cycles of PCR overlap extension, shPLP215 II was added and PCR amplification was continued for 35 cycles. The PCR product was cloned into pRSET via NheI and HindIII, 3′ to its 6xHis tag. DNA sequence analysis with the pRSET primers confirmed the DNA sequence of shPLP215 as an open reading frame with the ATG of pRSET. Expression of pRSET/shPLP215 into E. coli (BL21-DE3) and purification of shPLP215 was as described above.
T cell lines and T cell proliferative responses.
The pMOG35-55–specific T cell line raised from pMOG35-55–immunized C3H.SW mice was as described (18). The PLP139-151–specific T cell line was obtained in the same way, by in vitro selection from lymph node cells (LNC) of SJL/J mice primed 9 days previously with 100 μg PLP139-151 in CFA containing 250 μg Mycobacterium tuberculosis (Mt) H37Ra (3114-25; Difco Laboratories, Detroit, Michigan, USA). T cells specific for hmTAP were selected in vitro from LNCs of (C3H.SW × SJL/J)F1 mice primed 9 days previously with 200 μg hmTAP in CFA containing 150 μg Mt. All T cell lines were maintained in vitro in medium containing IL-2 with stimulation with the relevant antigen (10 μg/ml) in the presence of irradiated (25 Gy) syngeneic spleen cells (APCs) every 10–14 days as described (19).
Antigen-specific proliferative responses of line T cells (1.5 × 104/well) cultured in microtiter wells in the presence of irradiated syngeneic spleen cells as APCs (5 × 105/well) were assayed as described previously (19).
EAE induction.
Mice were injected subcutaneously in the flank with antigen emulsified in Mt-supplemented CFA (see figure legends for details) for active EAE. Adoptive transfer EAE experiments were conducted as described previously (18, 19). Briefly, line T cells stimulated in vitro with the relevant antigen were injected in 0.5 ml PBS 4 days later in the tail vein of naive syngeneic recipients. Mice were followed and scored daily on a scale of 0–6 as described previously (20).
Cytokine assays.
Cytokines were detected by ELISA according to PharMingen standard protocols (San Diego, California, USA). The capture Ab’s were rat anti-mouse IL-4 (18191D; PharMingen), rat anti-mouse IL-2 (18161D; PharMingen), rat anti-mouse IL-10 (AMC0102; BioSource International, Camarillo, California, USA) and rat anti-mouse IFN-γ (AMC4834; BioSource International). The biotinylated Ab’s were rat anti-mouse IL-2 (18172D; PharMingen), rat anti-mouse IL-4 (18042D; PharMingen), rat anti-mouse IL-10 (18152D; PharMingen), and rat anti-mouse IFN-γ (18112D; PharMingen).
Results
Construction of shMultiTAG/E and expression of hmTAP.
The shMultiTAG/E was designed to encode a pilot protein (hmTAP) to be used in investigating the feasibility of multi-autoantigen/multiepitope–directed immunomodulation of T cell–mediated autoimmune inflammatory diseases of the CNS. Because the immunomodulatory effect of hmTAP is to be tested in EAE associated with multiple pathogenic autoreactivities in (C3H.SW × SJL/J)F1 mice, the epitopes included for each of the potential encephalitogenic target antigens, MBP, PLP, or MOG, were selected according to their encephalitogenic potential in the parental mouse strains. Regions reported to contain major immunodominant epitopes recognized by T cells from patients with MS were also included in anticipation of future studies with epitope-specific T cells from MS patients. Accordingly, the epitope clusters selected for MOG included amino acids 32–58, which encompass the encephalitogenic region for H-2b mice (18), and amino acids 1–25 and 63–97, which represent, in addition to amino acids 32–58, regions containing immunodominant epitopes recognized by MS T cells (reviewed in ref. 1). The epitope clusters selected for MBP, at amino acids 7–50, 83–106, and 142–168, encompass the three major immunodominant regions most commonly recognized by T cells from patients with MS (reviewed in ref. 1), as well as the major encephalitogenic MBP epitope for SJL/J mice, MBP89-101 (reviewed in ref. 2), which is also encephalitogenic for H-2b mice (21). The epitope clusters selected for PLP contain the major immunodominant encephalitogenic epitope for SJL/J mice, PLP139-151 (reviewed in ref. 2), as well as epitopes reported to be frequently recognized by MS T cells, within amino acids 40–60, 94–117, and 138–155 (reviewed in refs. 22, 23).
ShTAG/MOG, shTAG/MBP, and shTAG/PLP minigenes each designed to encode for a polypeptide comprising the amino acids encompassed by the selected tandemly arranged regions of MOG, MBP, and PLP, respectively (Figure 1a), were generated as described in Methods. Each shTAG DNA subcloned into pRSET was transformed into E. coli for bacterial expression of the protein product. shMOG/E, strongly expressed from pRSET/shTAG/MOG, was purified by metal chelate chromatography as described in Methods. Because shMBP/E and shPLP/E could not be expressed from their respective pRSET/shTAGs, shPLP215 and rhMBP, which also contain the epitopes encoded by shTAG/PLP and shTAG/MBP, respectively, were generated as described in Methods.
Figure 1.
Generation of shMultiTAG/E coding for hmTAP. (a) Scheme for the construction of shMultiTAG/E. (b) ShMultiTAG/E DNA sequence and derived amino acid sequence of hmTAP.
The shMultiTAG/E was generated as described in Methods by ligating shTAG/MOG DNA, shTAG/MBP DNA, and shTAG/PLP DNA. The shMultiTAG/E DNA cloned into pRSET was sequenced (Figure 1b) and transformed into E. coli. SDS-PAGE analyses of bacterial expression and purification of shMOG/E, shPLP215, and hmTAP are shown in Figure 2a.
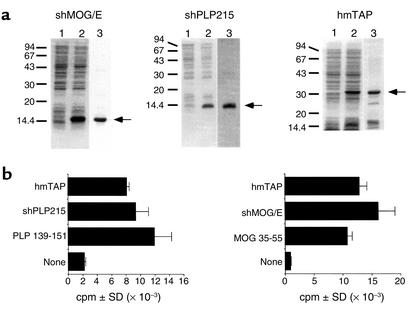
Figure 2.
Bacterial expression and purification of shMOG/E, shPLP215, and hmTAP, and their recognition by epitope-specific T cells. (a) Coomassie blue–stained SDS-PAGE analyses: lane 1, before IPTG induction; lane 2, after IPTG induction (15 μl, equivalent to 150 μl of bacterial culture); and lane 3, purified recombinant protein (5–10 μg). (b) Proliferative response by SJL/J PLP139-151–specific line T cells (left panel) and C3H.SW MOG 35-55–specific line T cells (right panel) to PLP139-151 (2.5 μg/ml), shPLP215 (10 μg/ml), or hmTAP (25 μg/ml), and to MOG35-55 (2.5 μg/ml), shMOG/E (10 μg/ml), or hmTAP (25 μg/ml), respectively.
HmTAP is adequately processed and presented to encephalitogenic epitope-specific line T cells.
Highly specific line T cells raised from PLP139-151–primed and MOG35-55–primed SJL/J and C3H.SW mice, respectively, were tested for their proliferative response to hmTAP. The PLP139-151–specific and the MOG35-55–specific line T cells reacted as strongly to hmTAP and to the relevant antigen, shPLP215 or shMOG/E, respectively, as they did to their stimulating synthetic peptide (Figure 2b). HmTAP could also be adequately processed in vivo because hmTAP-reactive T cells elicited upon immunization with hmTAP responded to shMOG/E, shPLP215, and rhMBP and peptides representing relevant epitopes thereof (see below). These results indicate that the epitopes within hmTAP are adequately processed and that their presentation to reactive T cells is not affected by their being joined together tandemly.
HmTAP abrogates the development of PLP139-151–induced EAE.
Because hmTAP can be adequately recognized by epitope-specific T cells in vitro, its potential effect in inhibiting such epitope-specific T cells in vivo was first assessed upon tolerogenic administration to SJL/J mice immunized for EAE induction with a single encephalitogenic peptide, PLP139-151, which is represented within hmTAP. From day 5 or 6 after the encephalitogenic challenge, the mice received on alternate days a total of four intraperitoneal injections of hmTAP, PLP139-151, or shMOG/E in PBS (Figure 3a). As can be seen in Figure 3a, shMOG/E had no suppressing effect on EAE induced with PLP139-151. Highly significant reduction in disease severity at the onset phase was observed in mice treated with the disease-relevant peptide, PLP139-151; however, such treatment did not prevent later recrudescence of disease, probably related to autoimmune spreading (Figure 3a). In contrast, intraperitoneal injections with hmTAP had a strong suppressive effect on PLP139-151–induced EAE, not only at the initial phase but also at the chronic stage of the disease (Figure 3a).
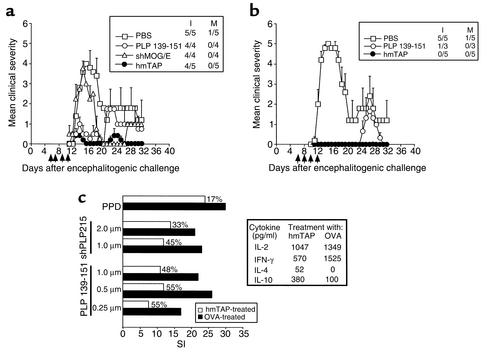
Figure 3.
Abrogation of PLP139-151–induced EAE by hmTAP is associated with inhibition of encephalitogenic T cells. Systemic administration of hmTAP intraperitoneally (a) and intravenously (b) suppresses PLP139-151–induced EAE. At the times indicated (arrows), SJL/J mice injected for EAE with PLP139-151 (150 μg) in CFA supplemented with 200 μg Mt, received 200 μg of PLP139-151, shPLP215, shMOG/E, or hmTAP in 500 μl PBS or PBS alone. I and M indicate incidence of disease and mortality in the group, respectively. (c) Inhibition of encephalitogenic T cells in hmTAP-treated mice is accompanied by a shift to noninflammatory cytokine secretion. Left panel: SJL/J mice primed with PLP139-151/CFA were injected intravenously on days 2, 4, 6, and 8 with hmTAP or OVA (∼100 μg) in 500 μl PBS. On day 9, isolated LNCs were analyzed for their proliferative response to PLP139-151 and shPLP215, as well as to purified protein derivative (PPD) of Mt (0.5 μg/200 μl culture) as a control for immunocompetence. Each histogram represents the mean stimulation index (SI) of triplicate cultures; the percentage of inhibition of proliferation by LNCs from hmTAP-treated mice as compared with LNCs from OVA-treated mice is indicated. Background cpm ± SD was 537 ± 100 and 432 ± 68 for LNCs isolated from mice injected with OVA and hmTAP, respectively. Right panel: Spleen cells from the hmTAP- or OVA-treated mice were incubated (5 × 106 spleen cells/ml) with PLP139-151 (20 μg/ml); IL-2 and IFN-γ were assayed by ELISA on supernatants collected after 12 hours in culture and IL-4 and IL-10 on supernatants collected after 72 hours in culture.
The effect of intravenous administration of hmTAP on PLP139-151–induced EAE in SJL/J mice was even more dramatic (Figure 3b). Injection of hmTAP in PBS in the tail vein on alternate days, from 5 or 6 days after encephalitogenic challenge until disease onset, fully abrogated PLP139-151–induced EAE (Figure 3b). In contrast, while the same regimen of intravenous injections with PLP139-151 also completely abrogated the initial phase, it did not prevent the development of symptoms at the expected relapse phase (Figure 3b). These results suggest that systemic administration of soluble hmTAP can suppress not only disease initiation, but also the development of neoreactivities that might be associated with disease chronicity. It also demonstrates how potent hmTAP can be as a therapeutic agent; indeed, while similar amounts of PLP139-151 and hmTAP were administered, the molar ratio of PLP139-151 integral to hmTAP, as compared to the synthetic peptide itself, is approximately 1:20.
Inhibition of encephalitogenic T cells in hmTAP-treated mice.
Lymph node cells of mice immunized for EAE with PLP139-151 and that had received intravenous injections of hmTAP or ovalbumin (OVA) in PBS were analyzed for their proliferative response to PLP139-151 or shPLP215. As can be seen in Figure 3c (left panel), while the reactivity to PPD did not differ in the hmTAP- and OVA-injected mice, indicating that the general immunocompetence was not affected, the responses of elicited PLP139-151–reactive T cells to their specific antigen were inhibited by approximately 50% in hmTAP-treated mice as compared with the control OVA-injected mice. Data from cytokine analyses (Figure 3c, right panel) suggest that the markedly reduced proliferation to the encephalitogenic epitope in hmTAP-treated mice, as compared with OVA-treated mice, was accompanied by a decrease in secretion of INF-γ, in parallel with an increase in secretion of IL-4 and IL-10 by spleen cells from the same animals. No significant difference in IL-2 secretion was observed.
HmTAP inhibits EAE transferred by epitope-specific committed T cells.
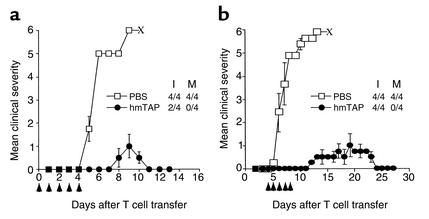
In the context of the MS model, it would be more relevant to inhibit already committed pathogenic T cells, such as would be expected in MS, using the passive EAE model. Accordingly, the potential protective and/or curative effect of tolerogenically administered hmTAP was tested on EAE passively transferred by activated PLP139-151–specific line T cells. Naive SJL/J mice were injected intravenously with PLP139-151–specific line T cells. From day 0 to day 4 the mice received a single daily injection intravenously with hmTAP or PBS alone (Figure 4a). While the four control PBS-injected mice developed very severe disease, of which all had died within 9 days after T cell transfer, tolerogenic administration of hmTAP was remarkably effective in preventing disease development consecutive to adoptive transfer with PLP139-151–specific line T cells (Figure 4a). Similarly, when treatment with hmTAP was initiated at expected disease onset, the hmTAP-treated mice suffered only mild disease from which they fully recovered within 10–12 days after disease onset (Figure 4b). All four control PBS-injected mice had died of severe EAE within a week after disease onset. These data suggest that hmTAP is as effective in inhibiting the disease-causing activity of already committed encephalitogenic T cells (Figure 4a) as in suppressing their development in actively induced EAE (Figure 3, a and b) and can actually arrest ongoing disease mediated by such cells (Figure 4b).
Figure 4.
HmTAP suppresses and treats EAE transferred with PLP139-151–specific line T cells. Naive SJL/J mice injected intravenously on day 0 with 5 × 105 activated PLP139-151–specific line T cells received a daily intravenous injection (arrows) of approximately 100 μg hmTAP in 500 μl PBS or PBS alone (a) from day 0 (12 hours after the T cell transfer) to day 4 for suppression of EAE, or (b) from day 4 (anticipated day of disease onset) to day 8 for treatment of EAE.
Concomitant multiepitope-directed targeting inhibits development of EAE associated with multiple pathogenic autoreactivities.
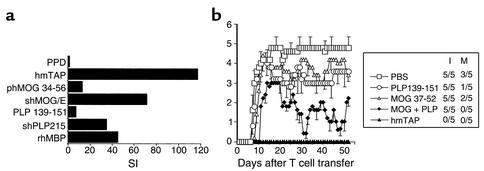
Previous studies (15, 16) have suggested that targeting autoreactivity against a single encephalitogenic protein/epitope is not a sufficiently effective approach to immunomodulation of EAE induced by two encephalitogenic proteins/epitopes. To investigate the feasibility and efficacy of multiepitope targeting as an approach to immunomodulation of EAE associated with multiple primary pathogenic autoreactivities using the pilot “multiple epitope-encompassing” hmTAP, a new model of complex EAE was established in (C3H.SW × SJL/J)F1 mice. These mice develop severe EAE upon active immunization with any shMOG/E, shPLP215, or rhMBP, with PLP139-151 or MOG35-55 encephalitogenic for the parental SJL/J and C3H.SW mice, respectively, and with hmTAP (data not shown). To better simulate the situation in MS where committed, potentially pathogenic T cells are already present, adoptive transfer, rather than active immunization, was selected for disease induction. To obtain a disease associated with multiple defined autoreactivities, the (C3H.SW × SJL/J)F1 mice were transferred with hmTAP-reactive line T cells selected from lymph node cells isolated from (C3H.SW × SJL/J)F1 mice primed with hmTAP. As shown in Figure 5a, after four cycles of in vitro selection with hmTAP, hmTAP-reactive line T cells were multispecific, showing sustained response to rhMBP, shPLP215, and shMOG/E and encephalitogenic epitopes thereof (stimulation indices ranging from about 10 to almost 80; Figure 5a). Adoptive transfer of these multispecific line T cells into syngeneic recipients induced a severe EAE (Figure 5b), apparently associated with multiple pathogenic autoreactivities. As shown in Figure 5b, this complex EAE could not be suppressed by tolerogenic administration (intraperitoneal injection daily from day 1 to day 9) of single peptides representing encephalitogenic epitopes, PLP139-151 or MOG37-52; a mixture of these two peptides had no effect on disease incidence, albeit reduced disease severity (Figure 5b). A dramatic, long-lasting full abrogation of disease development was, however, observed upon treatment with hmTAP according to the same regimen (Figure 5b).
Figure 5.
Concomitant multiepitope-directed targeting inhibits development of EAE associated with multiple pathogenic autoreactivities. (a) Multiantigen/epitope reactivity of hmTAP-reactive line T cells. Line T cells selected in vitro with hmTAP from LNCs of (C3H.SW × SJL/J)F1 mice immunized with hmTAP/CFA were analyzed after four rounds of selection for their proliferative response to hmTAP, shMOG/E, rhMBP, shPLP215, and relevant encephalitogenic peptides (10 μg/ml). The proliferative response to PPD (5 μg/ml) was analyzed as a measure of specificity. (b) Tolerogenic administration of hmTAP completely abrogates the development in (C3H.SW × SJL/J)F1 mice of EAE adoptively transferred by multispecific hmTAP-reactive line T cells. On day 0 (C3H.SW × SJL/J)F1 mice were injected intravenously with the multispecific hmTAP-reactive line T cells (2 × 106 cells). From day 1 to day 9 after T cell transfer the mice were injected daily intraperatoneally with PLP139-151 (150 μg), MOG37-52 (150 μg), or a mixture of both MOG35-55 and PLP139-151 (MOG + PLP; each 150 μg), or with hmTAP (100 μg), or with PBS alone (500 μl).
Discussion
The studies presented here demonstrate that a multiantigen/multiepitope artificial protein could be specifically designed for immune-specific modulation of multiple pathogenic autoreactivities associated with autoimmune disease. A prerequisite to a potential immunomodulatory activity of the pilot multiple epitope-encompassing hmTAP was the integrity of relevant epitopes upon in vitro and in vivo antigenic processing. As demonstrated, the junctions between hmTAP-integral epitopes did not interfere with their antigenic processing and presentation: line T cells raised to peptides representing the different relevant encephalitogenic epitopes reacted with hmTAP, and hmTAP-reactive T cells elicited in vivo could be stimulated by peptides representing distinct encephalitogenic epitopes. The appropriate in vitro and in vivo “recognition” of MOG, MBP, and PLP epitopes integral to hmTAP suggests that hmTAP could be effective in targeting the relevant encephalitogenic epitope-specific T cells in vivo. As expected, systemic administration of hmTAP was highly effective in abrogating EAE actively induced by a peptide representing a single defined encephalitogenic epitope present in hmTAP, PLP139-151; it also evidently targeted emerging neoreactivities leading to disease chronicity, because clinical symptoms were not observed at the expected time of disease relapse. HmTAP was also highly effective in neutralizing already committed encephalitogenic T cells because it not only prevented the development of EAE passively transferred with PLP139-151–specific T cells, but also arrested the ongoing disease. Most importantly, and of high relevance to therapy of MS and other human T cell–mediated autoimmune diseases, which are apparently already associated with multiple pathogenic T cell autoreactivities at the time of diagnosis, systemically administered hmTAP fully prevented the development of a complex EAE associated with concomitant pathogenic autoreactivities against MBP, PLP, and MOG induced by line T cells raised to hmTAP. In contrast, targeting a single autoreactivity by tolerization with individual peptides had no significant effect on this complex EAE.
The mechanism(s) by which hmTAP suppresses actively induced or passively transferred EAE have not been fully investigated. As demonstrated in other studies, various mechanisms may be involved in peripheral tolerance upon systemic administration of soluble antigen. Clonal deletion or functional inactivation (anergy) of antigen-specific T cells (24–26) and immune deviation to an anti-inflammatory Th2 phenotype by unresponsiveness in Th1 but not Th2 cells (24, 27) or inactivation of pathogenic T cells and production of anti-inflammatory cytokines as a result of antigen presentation via peripheral APCs, which do not express optimal costimulatory molecules (28), have been postulated as possible mechanisms underlying peripheral antigenic tolerization. Upregulation of regulatory T cells upon intravenous administration of autoantigen has also been suggested (29), and these may in turn secrete immunosuppressive cytokines (30, 31). Another type of regulation of the immune response may be mediated by antigen-specific T cells anergized by intravenous administration of the relevant antigen, which can act as suppressor cells (32). The demonstration that abrogation of PLP139-151–induced EAE in mice tolerized with hmTAP correlated with inhibition of PLP-specific proliferative T cell response and expression of an anti-inflammatory cytokine pattern is consistent with any one or a combination of these mechanisms. However, it is likely that the suppressive mechanisms and their modes of action are different in actively induced and passively transferred EAE, and further investigations are necessary to determine the precise mechanism(s) operating. Regardless, systemic administration of hmTAP offers long-lasting protection, apparently by neutralizing the multiple concomitant pathogenic autoreactivities resulting in EAE.
In MS, the multiplicity of potentially pathogenic autoreactivities against the myelin components, MBP, PLP, MOG (reviewed in ref. 1), myelin-associated oligodendrocytic basic protein (3, 4), and oligodendrocyte-specific protein (7), which have been detected in different patients, suggest that the primary antigen(s) and/or the major epitope(s) against which the dominant pathogenic autoreactivities are directed may differ in different patients. Because neoreactivities are also likely to emerge (13, 14), disease progression may be associated with multiple potentially pathogenic T cell autoreactivities. Studies aimed at inhibiting the development or progression of EAE associated with more than one pathogenic autoreactivity by antigen-specific immunomodulation targeting a single specificity have not yielded significant results, but rather have suggested that all relevant autoreactivities should be targeted for maximal therapeutic effect (15, 16). The results obtained in the present study fully corroborate these observations. Thus, when disease was initiated by autoreactivities to several encephalitogenic epitopes concomitantly, neutralization of only a single autoreactivity had little effect on overall disease expression. Regardless of whether the disease was actively induced by immunization with three encephalitogenic epitopes (data not shown) or passively transferred by multispecific T cells, effective disease suppression was achieved only by multiepitope targeting. In MS, two clinical trials based on targeting a single epitope of a single candidate autoantigen were recently conducted (33, 34). Although terminated prematurely, these phase II clinical trials that involved subcutaneous injection of the same altered peptide ligand (APL) of MBP83-99, i.e., MBP83-99 substituted at residues 83, 84, 89, and 91, did not show convincing therapeutic benefit. The report of Bielekova et al. (33) also indicated a potential risk of exacerbation associated with stimulation of MBP-reactive T cells in patients treated with a high dosage of MBP-APL, while such an observation was not made in the large clinical trial including patients receiving low-dose APL treatment (34). Among possible reasons for the lack of significant clinical improvement in “low dose–treated” patients (34), the complexity of antimyelin autoreactivities may be a major factor. An anticipated bystander effect of the MBP-APL treatment was only marginal (33, 34). These observations may therefore be in line with data obtained in the animal model. In complex EAE, effective peripheral tolerization is epitope specific, and each of the multiple immunodominant pathogenic autoreactivities requires tolerization for optimal efficacy of antigen-specific therapeutic approaches. In this context, the present study and that reported by Elliott et al. (16) used artificial proteins to counteract multiple pathogenic autoreactivities in EAE by a single immunomodulatory agent. Systemic administration of MP4 (21.5-kDa MBP isoform fused to ΔPLP) prepared by Elliott et al. suppressed EAE transferred by T cells reactive to MBP and PLP and effectively treated EAE transferred by PLP-specific T cells (16). The hmTAP, encompassing genetically ligated disease-relevant epitopes of MBP, PLP, and MOG, abrogated the development of complex EAE transferred by multispecific line T cells reactive to MBP, PLP, and MOG. In both studies, targeting only a single antigen/epitope had no significant therapeutic benefit on EAE associated with multiple pathogenic autoreactivities.
The results obtained with the artificially designed hmTAP in the experimental model suggest that multiantigen/multiepitope–targeted immunomodulation should be further explored as a conceptual approach to therapy for MS and other organ-specific T cell–mediated human autoimmune diseases. Studies are in progress toward constructing a synthetic gene coding for MS-related (HLA-DR2–restricted) T cell epitopes of the known encephalitogens MBP, PLP, MOG, myelin-associated oligodendrocyte basic protein, and oligodendrocyte-specific protein and investigating its protein product for induction of peripheral tolerance against the multiple relevant T cell autoreactivities in appropriate animal models. In this context, the protein products of synthetic genes specifically designed to encode nonstimulatory antagonistic analogues of disease-related T cell epitopes may be more applicable for eventual therapy.
Acknowledgments
This work was supported in part by grants from the National Multiple Sclerosis Society of New York (RG3195A7/1), the Israel Science Foundation, and the Crown Endowment Fund for Immunological Research. A. Ben-Nun is the incumbent of the Eugene and Marcia Appelbaum Professorial Chair.
Footnotes
Conflict of interest: No conflict of interest has been declared.
Nonstandard abbreviations used: Multiple sclerosis (MS); myelin basic protein (MBP); proteolipid protein (PLP); myelin oligodendrocyte glycoprotein (MOG); experimental autoimmune encephalomyelitis (EAE); human multitarget autoantigen protein (hmTAP); ovalbumin (OVA); altered peptide ligand (APL); lymph node cell (LNC).
References
- 1.Kerlero de Rosbo N, Ben-Nun A. T-cell responses to myelin antigens in multiple sclerosis: relevance of the predominant autoimmune reactivity to myelin oligodendrocyte glycoprotein. J Autoimmun. 1998;11:287–299. doi: 10.1006/jaut.1998.0202. [DOI] [PubMed] [Google Scholar]
- 2.Kerlero de Rosbo, N., and Ben-Nun, A. 1999. Experimental autoimmune encephalomyelitis induced by various antigens of the central nervous system. Overview and relevance to multiple sclerosis. In The decade of autoimmunity 1987–1997. Y. Schoenfeld, editor. Elsevier Science. Amsterdam, The Netherlands. 169–177.
- 3.Holz A, Bielekova B, Martin R, Oldstone MBA. Myelin-associated oligodendrocytic basic protein: identification of an encephalitogenic epitope and association with multiple sclerosis. J Immunol. 2000;164:1103–1109. doi: 10.4049/jimmunol.164.2.1103. [DOI] [PubMed] [Google Scholar]
- 4.Kaye J, et al. The central nervous system-specific myelin oligodendrocytic basic protein (MOBP) is encephalitogenic and a potential target antigen in multiple sclerosis. J Neuroimmunol. 2000;102:189–198. doi: 10.1016/s0165-5728(99)00168-x. [DOI] [PubMed] [Google Scholar]
- 5.Stevens DB, Chen K, Seitz RS, Sercarz EE, Bronstein JM. Oligodendrocyte-specific protein peptides induce experimental autoimmune encephalomyelitis in SJL/J mice. J Immunol. 1999;162:7501–7509. [PubMed] [Google Scholar]
- 6.Zhong M-C, Cohen L, Meshorer A, Kerlero de Rosbo N, Ben-Nun A. T-cells specific for soluble recombinant oligodendrocyte-specific protein induce severe experimental autoimmune encephalomyelitis in H-2b and H-2smice. J Neuroimmunol. 2000;105:39–45. doi: 10.1016/s0165-5728(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 7.Vu T, Myers LW, Ellison GW, Mendoza F, Bronstein JM. T-cell responses to oligodendrocyte-specific protein in multiple sclerosis. J Neurosci Res. 2001;66:506–509. doi: 10.1002/jnr.1241. [DOI] [PubMed] [Google Scholar]
- 8.McCarron RM, Fallis RJ, McFarlin DE. Alterations in T cell antigen specificity and class II restriction during the course of chronic relapsing experimental allergic encephalomyelitis. J Neuroimmunol. 1990;29:73–79. doi: 10.1016/0165-5728(90)90149-h. [DOI] [PubMed] [Google Scholar]
- 9.Perry LL, Bargaza-Gilbert E, Trotter JL. T cell sensitization to proteolipid protein in myelin basic protein-induced relapsing experimental allergic encephalomyelitis. J Neuroimmunol. 1991;33:7–15. doi: 10.1016/0165-5728(91)90029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehman PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 11.McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu M, Johnston JM, Tuohy VK. A predictable sequential determinant spreading cascade invariably accompanies progression of experimental autoimmune encephalomyelitis: a basis for peptide-specific therapy after onset of clinical disease. J Exp Med. 1996;183:1777–1788. doi: 10.1084/jem.183.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuohy VK, Yu M, Yin L, Kawczak JA, Kinkel RP. Spontaneous regression of primary autoreactivity during chronic progression of experimental autoimmune encephalomyelitis and multiple sclerosis. J Exp Med. 1999;189:1033–1042. doi: 10.1084/jem.189.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuohy VK, Yu M, Weinstock-Guttman B, Kinkel RP. Diversity and plasticity of self recognition during the development of multiple sclerosis. J Clin Invest. 1997;99:1682–1690. doi: 10.1172/JCI119331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leadbetter EA, et al. Experimental autoimmune encephalomyelitis induced with a combination of myelin basic protein and myelin oligodendrocyte glycoprotein is ameliorated by administration of a single myelin basic protein peptide. J Immunol. 1998;161:504–512. [PubMed] [Google Scholar]
- 16.Elliott EA, et al. Treatment of experimental encephalomyelitis with a novel chimeric fusion protein of myelin basic protein and proteolipid protein. J Clin Invest. 1996;98:1602–1612. doi: 10.1172/JCI118954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamholz J, de Ferra F, Puckett C, Lazzarini R. Identification of three forms of human myelin basic protein by cDNA cloning. Proc Natl Acad Sci USA. 1986;83:4962–4966. doi: 10.1073/pnas.83.13.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendel I, Kerlero de Rosbo N, Ben-Nun A. Delineation of the minimal encephalitogenic epitope within the immunodominant region of myelin oligodendrocyte glycoprotein. Diverse Vb gene usage by T cells recognizing the core epitope encephalitogenic for TCR-Vbb and TCR-Vba H-2bmice. Eur J Immunol. 1996;26:2470–2479. doi: 10.1002/eji.1830261030. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Nun A, Lando Z. Detection of cells responding to myelin basic protein by proliferation and selection of T cell lines functional in mediating experimental autoimmune encephalomyelitis in mice. J Immunol. 1983;130:1205–1209. [PubMed] [Google Scholar]
- 20.Ben-Nun A, Yossefi S, Lehman D. Protection against autoimmune disease by bacterial agents. II. PPD and pertussis toxin as proteins active in protecting mice against experimental autoimmune encephalomyelitis. Eur J Immunol. 1993;23:689–696. doi: 10.1002/eji.1830230318. [DOI] [PubMed] [Google Scholar]
- 21.Fritz RB, Zhao M-L. Active and passive experimental autoimmune encephaloimyelitis in strain 129/J (H-2b) mice. J Neurosci Res. 1996;45:471–474. doi: 10.1002/(SICI)1097-4547(19960815)45:4<471::AID-JNR17>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Tuohy VK. Peptide determinants of myelin proteolipid protein (PLP) in autoimmune disease: a review. Neurochem Res. 1994;19:935–944. doi: 10.1007/BF00968703. [DOI] [PubMed] [Google Scholar]
- 23.Trotter JL, et al. T cell recognition of myelin proteolipid protein and myelin proteolipid protein peptides in the peripheral blood of multiple sclerosis and control subjects. J Neuroimmunol. 1998;84:172–178. doi: 10.1016/s0165-5728(97)00260-9. [DOI] [PubMed] [Google Scholar]
- 24.Burstein HJ, Abbas AK. In vivo role of interleukin 4 in T cell tolerance induced by aqueous protein antigen. J Exp Med. 1993;177:457–463. doi: 10.1084/jem.177.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liblau RS, et al. Intravenous injection of soluble antigen induces thymic and peripheral T-cell apoptosis. Proc Natl Acad Sci USA. 1996;93:3031–3036. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Critchfield JM, et al. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 27.Burstein HJ, Shea CM, Abbas AK. Aqueous antigens induce in vivo tolerance selectively in IL-2 and IFN-γ-producing (Th1) cells. J Immunol. 1992;148:3687–3691. [PubMed] [Google Scholar]
- 28.Legge KL, et al. Coupling of peripheral tolerance to endogenous interleukin 10 promotes effective modulation of myelin-activated T cells and ameliorates experimental allergic encephalomyelitis. J Exp Med. 2000;191:2039–2051. doi: 10.1084/jem.191.12.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilliard BA, Kamoun M, Ventura E, Rostami A. Mechanisms of suppression of experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein: role of regulatory spleen cells. Exp Mol Pathol. 2000;68:29–37. doi: 10.1006/exmp.1999.2290. [DOI] [PubMed] [Google Scholar]
- 30.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-β but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RBlow CD4+T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groux H, et al. A CD4+T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 32.Taams LS, Wauben MHM. Anergic T cells as active regulators of the immune response. Hum Immunol. 2000;61:633–639. doi: 10.1016/s0198-8859(00)00127-0. [DOI] [PubMed] [Google Scholar]
- 33.Bielekova B, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 34.Kappos L, et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat Med. 2000;6:1176–1182. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]