Abstract
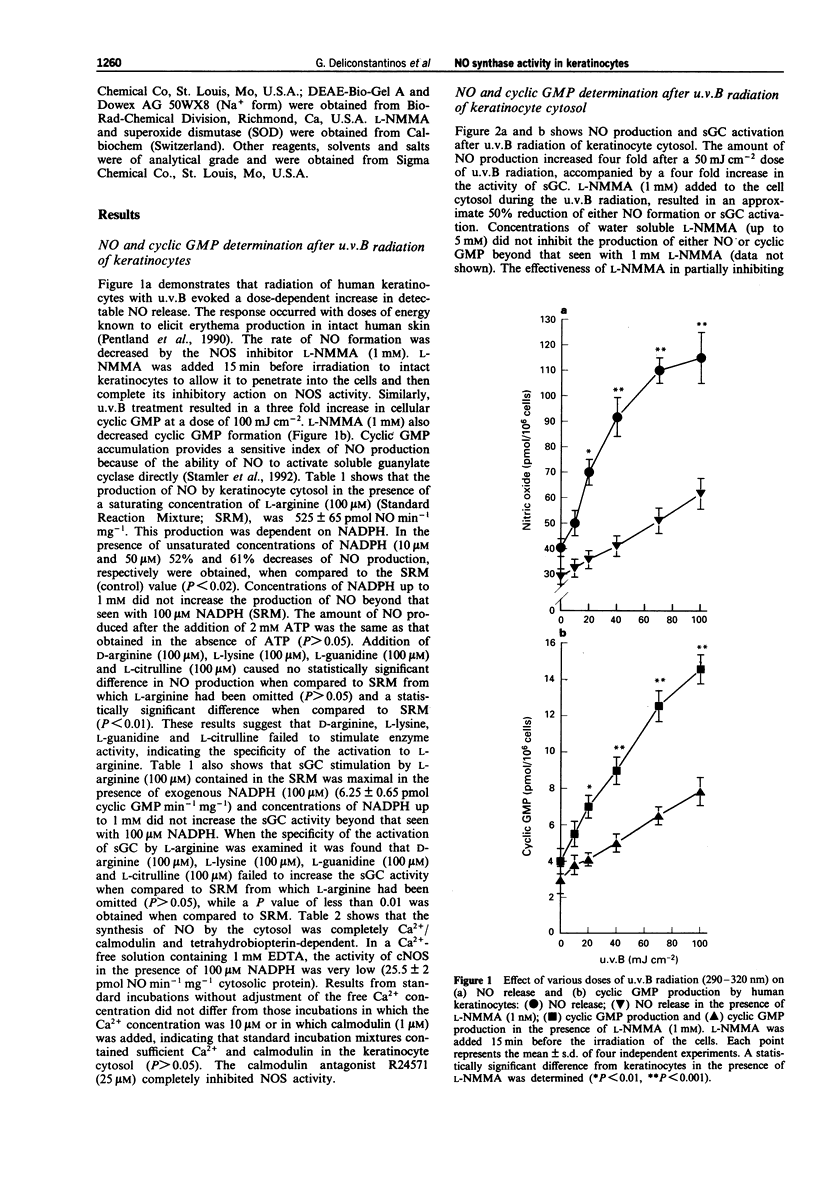
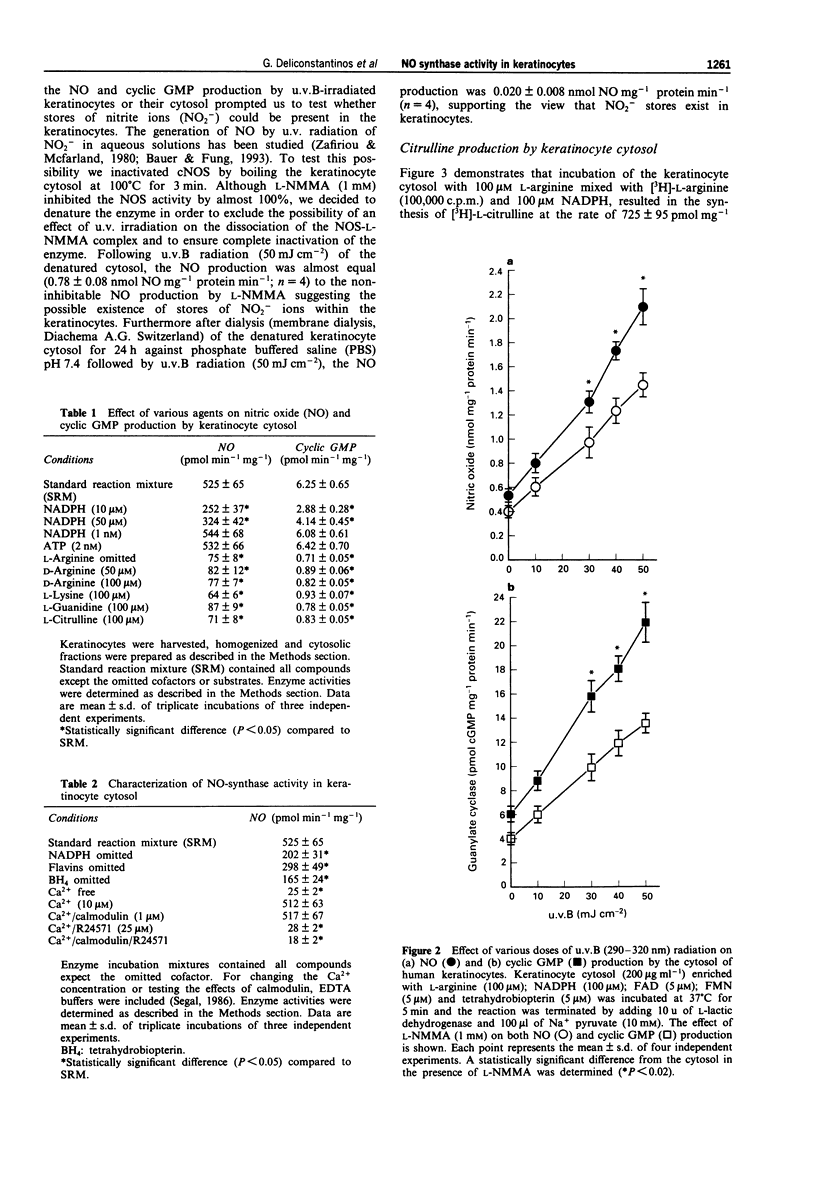
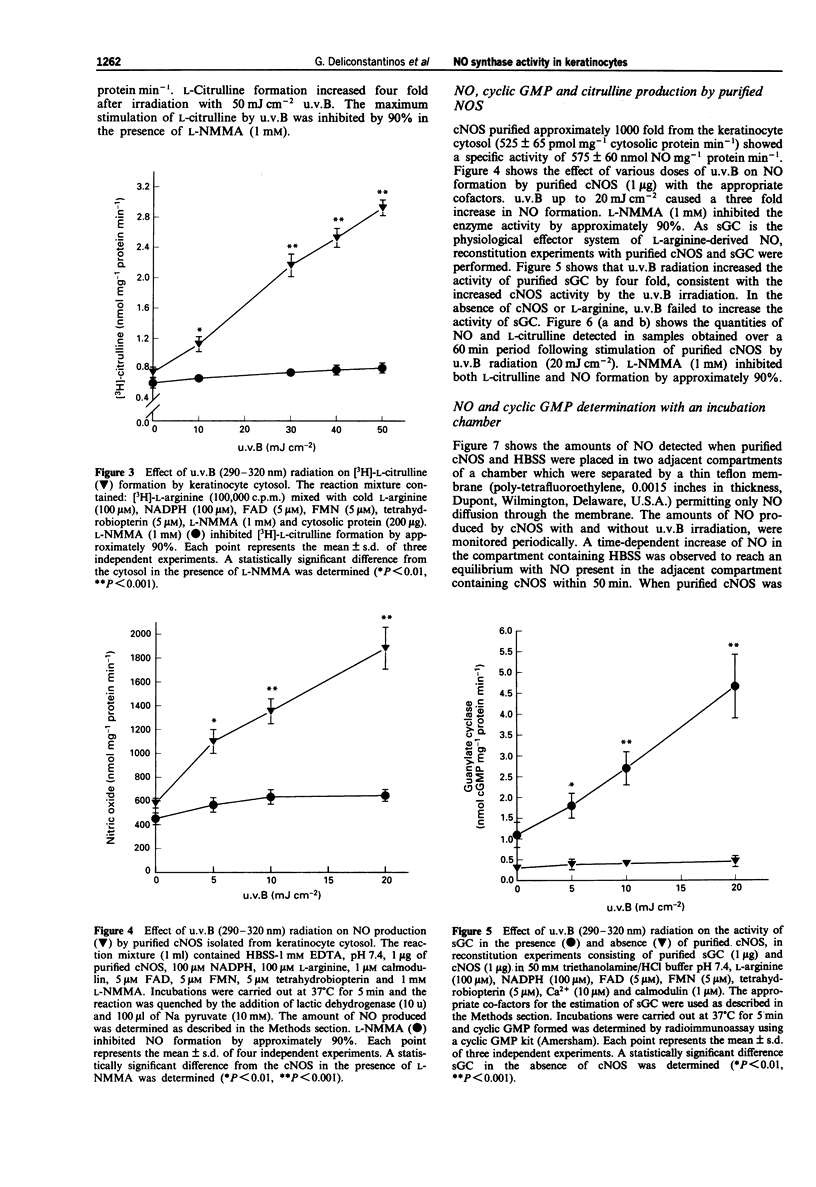
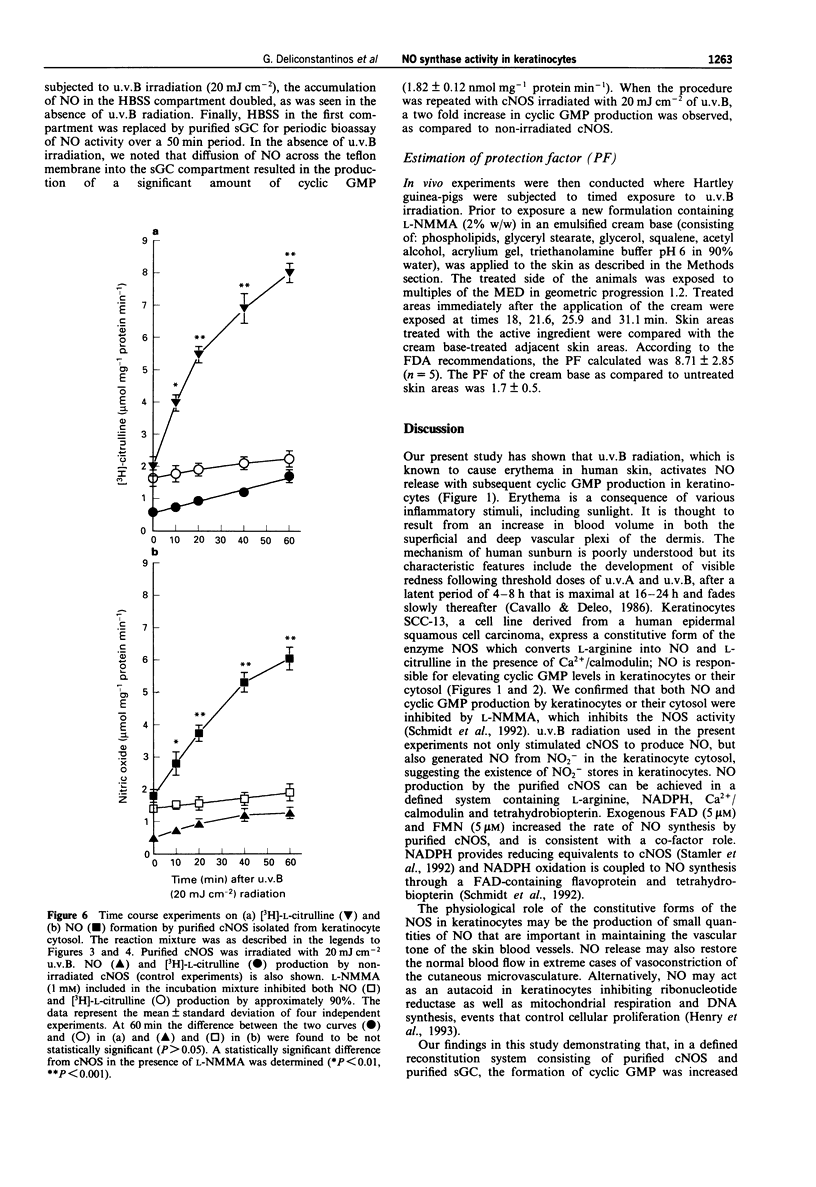
1. The mechanism of human sunburn is poorly understood but its characteristic features include the development of erythema. In this study we attempted to determine whether human keratinocytes possess a nitric oxide (NO) synthase (NOS), if this enzyme could be activated to release NO following exposure to ultraviolet B (u.v.B) and to define whether this photo-induced response could be involved in the pathogenesis of sunburn erythema. 2. Treatment of human keratinocytes with various doses of u.v.B (290-320 nm) radiation (up to 100 mJ cm-2) resulted in a dose-dependent release of NO and cyclic GMP production that was reduced by NG-monomethyl-L-arginine (L-NMMA). 3. u.v.B irradiation of keratinocyte cytosol at varying doses (up to 50 mJ cm-2), resulted in a gradual rise in NO production, with a concomitant increase in soluble guanylate cyclase activity (sGC). 4. NOS isolated from the keratinocyte cytosol was constitutively expressed and was dependent on NADPH, Ca2+/calmodulin, tetrahydrobiopterin and flavins. 5. In reconstitution experiments, when purified NOS was added to purified sGC, both isolated from keratinocyte cytosol, a four fold increase in cyclic GMP was observed. The GMP was increased by NO synthesized following u.v.B radiation (up to 20 mJ cm-2) of NOS. 6. In in vivo experiments, guinea-pigs were subjected to u.v.B light. A Protection Factor (PF) of 8.71 +/- 2.85 was calculated when an emulsified cream formulation containing L-NMMA (2%) was applied to their skin. 7. The present results indicate that u.v.B radiation acts as a potent stimulator of NOS in keratinocytes.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


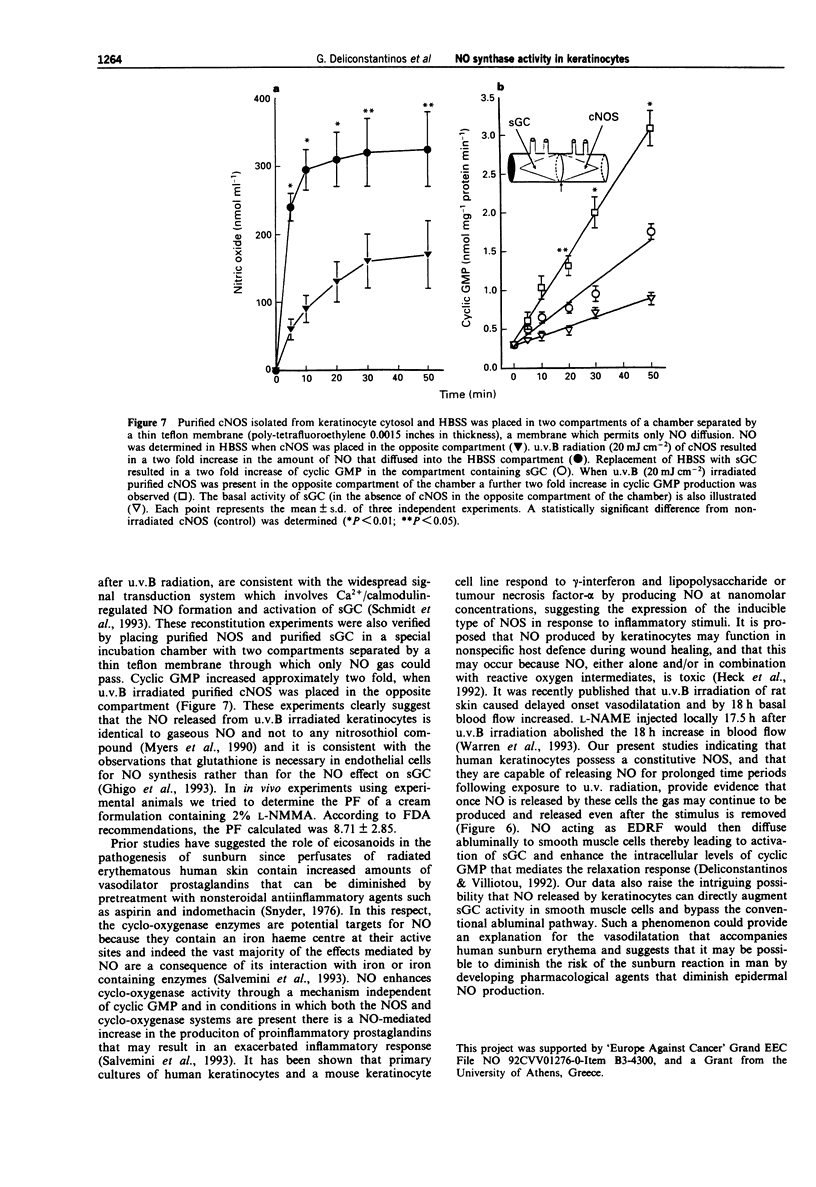

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990 Jun 4;265(1-2):133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- Cavallo J., DeLeo V. A. Sunburn. Dermatol Clin. 1986 Apr;4(2):181–187. [PubMed] [Google Scholar]
- Deliconstantinos G., Villiotou V., Fassitsas C. Ultraviolet-irradiated human endothelial cells elaborate nitric oxide that may evoke vasodilatory response. J Cardiovasc Pharmacol. 1992;20 (Suppl 12):S63–S65. doi: 10.1097/00005344-199204002-00019. [DOI] [PubMed] [Google Scholar]
- Ghigo D., Alessio P., Foco A., Bussolino F., Costamagna C., Heller R., Garbarino G., Pescarmona G. P., Bosia A. Nitric oxide synthesis is impaired in glutathione-depleted human umbilical vein endothelial cells. Am J Physiol. 1993 Sep;265(3 Pt 1):C728–C732. doi: 10.1152/ajpcell.1993.265.3.C728. [DOI] [PubMed] [Google Scholar]
- Greaves M. W. Ultraviolet erythema: causes and consequences. Curr Probl Dermatol. 1986;15:18–24. doi: 10.1159/000412089. [DOI] [PubMed] [Google Scholar]
- Heck D. E., Laskin D. L., Gardner C. R., Laskin J. D. Epidermal growth factor suppresses nitric oxide and hydrogen peroxide production by keratinocytes. Potential role for nitric oxide in the regulation of wound healing. J Biol Chem. 1992 Oct 25;267(30):21277–21280. [PubMed] [Google Scholar]
- Henry Y., Lepoivre M., Drapier J. C., Ducrocq C., Boucher J. L., Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J. 1993 Sep;7(12):1124–1134. doi: 10.1096/fasebj.7.12.8397130. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacol Toxicol. 1990 Jul;67(1):1–7. doi: 10.1111/j.1600-0773.1990.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Keaney J. F., Jr, Simon D. I., Stamler J. S., Jaraki O., Scharfstein J., Vita J. A., Loscalzo J. NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J Clin Invest. 1993 Apr;91(4):1582–1589. doi: 10.1172/JCI116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiechle F. L., Malinski T. Nitric oxide. Biochemistry, pathophysiology, and detection. Am J Clin Pathol. 1993 Nov;100(5):567–575. doi: 10.1093/ajcp/100.5.567. [DOI] [PubMed] [Google Scholar]
- Lowenstein C. J., Dinerman J. L., Snyder S. H. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994 Feb 1;120(3):227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- Mayer B., Schmidt K., Humbert P., Böhme E. Biosynthesis of endothelium-derived relaxing factor: a cytosolic enzyme in porcine aortic endothelial cells Ca2+-dependently converts L-arginine into an activator of soluble guanylyl cyclase. Biochem Biophys Res Commun. 1989 Oct 31;164(2):678–685. doi: 10.1016/0006-291x(89)91513-1. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pentland A. P., Mahoney M., Jacobs S. C., Holtzman M. J. Enhanced prostaglandin synthesis after ultraviolet injury is mediated by endogenous histamine stimulation. A mechanism for irradiation erythema. J Clin Invest. 1990 Aug;86(2):566–574. doi: 10.1172/JCI114746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pufahl R. A., Marletta M. A. Oxidation of NG-hydroxy-L-arginine by nitric oxide synthase: evidence for the involvement of the heme in catalysis. Biochem Biophys Res Commun. 1993 Jun 30;193(3):963–970. doi: 10.1006/bbrc.1993.1719. [DOI] [PubMed] [Google Scholar]
- Salvemini D., Misko T. P., Masferrer J. L., Seibert K., Currie M. G., Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Lohmann S. M., Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993 Aug 18;1178(2):153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Werner E. R., Mayer B., Wachter H., Kukovetz W. R. Tetrahydrobiopterin-dependent formation of endothelium-derived relaxing factor (nitric oxide) in aortic endothelial cells. Biochem J. 1992 Jan 15;281(Pt 2):297–300. doi: 10.1042/bj2810297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J. Lanthanum increases the rat thymocyte cytoplasmic free calcium concentration by enhancing calcium influx. Biochim Biophys Acta. 1986 Apr 29;886(2):267–271. doi: 10.1016/0167-4889(86)90144-8. [DOI] [PubMed] [Google Scholar]
- Shikano K., Ohlstein E. H., Berkowitz B. A. Differential selectivity of endothelium-derived relaxing factor and nitric oxide in smooth muscle. Br J Pharmacol. 1987 Nov;92(3):483–485. doi: 10.1111/j.1476-5381.1987.tb11347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D. S. Effect of topical indomethacin on UVR-induced redness and prostaglandin E levels in sunburned guinea pig skin. Prostaglandins. 1976 Apr;11(4):631–643. doi: 10.1016/0090-6980(76)90066-6. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Warren J. B., Loi R. K., Coughlan M. L. Involvement of nitric oxide synthase in the delayed vasodilator response to ultraviolet light irradiation of rat skin in vivo. Br J Pharmacol. 1993 Jul;109(3):802–806. doi: 10.1111/j.1476-5381.1993.tb13645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


