Abstract
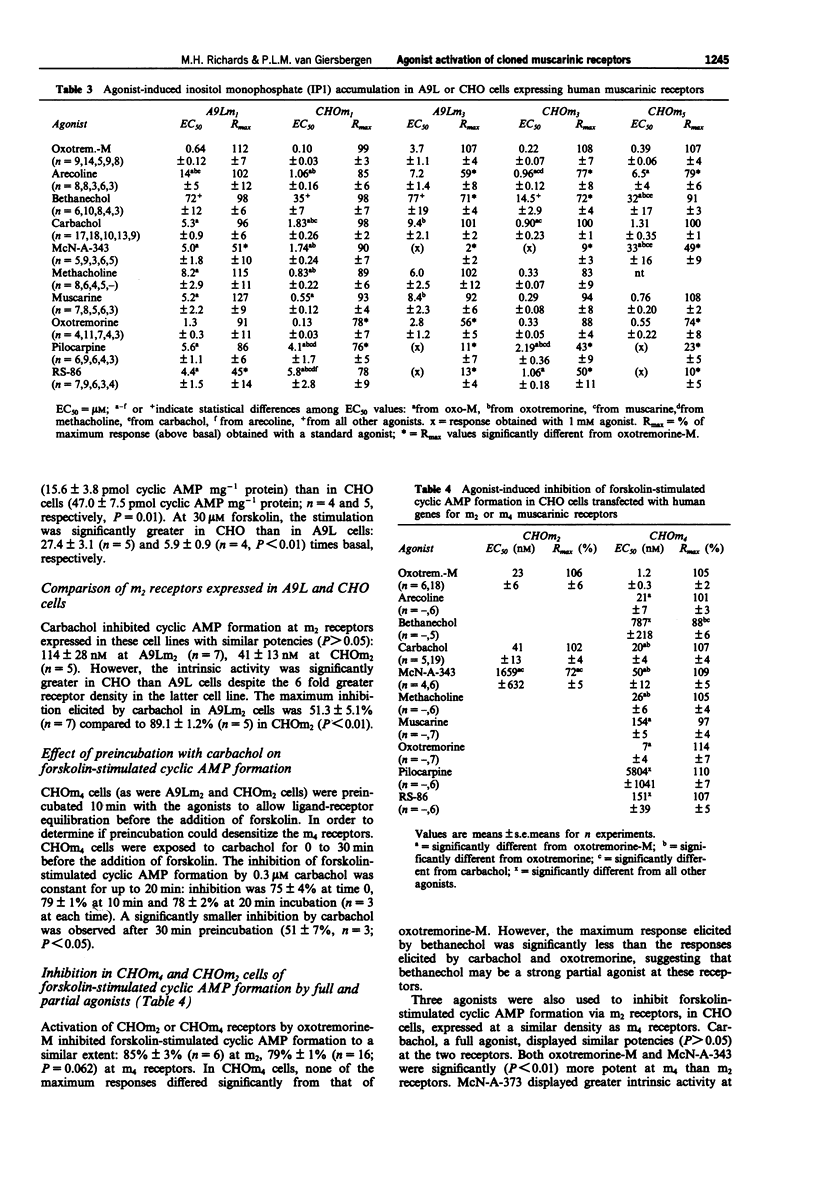
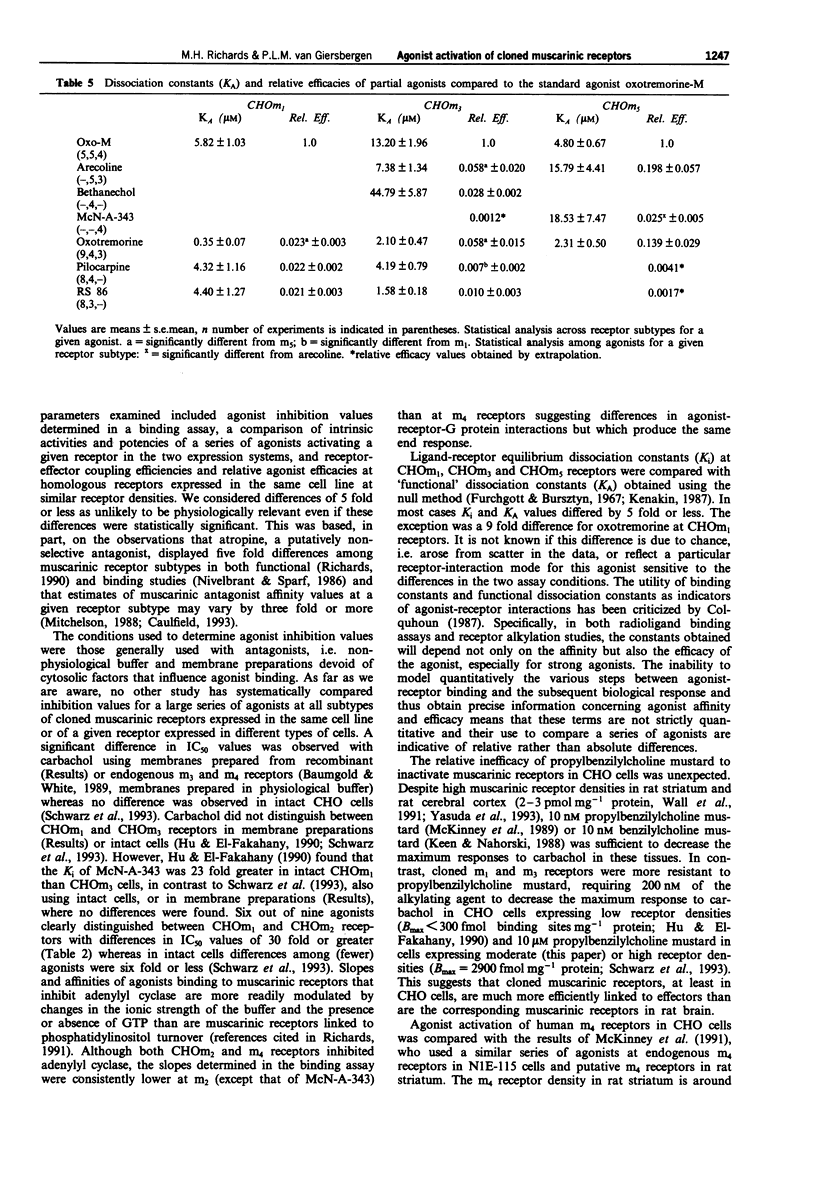
1. A comparative study of receptor activation by ten full and partial muscarinic agonists was undertaken on the five subtypes of human muscarinic receptors expressed at similar receptor densities in Chinese hamster ovary (CHO-K1) cells. In addition, m1, m2 and m3 receptors were expressed in mouse fibroblast A9L cells in order to compare the influences of cell type on agonist activation of these receptors. 2. Receptor-effector coupling efficiencies were greater in CHO than A9L cells and agonists displayed greater potencies and similar or greater intrinsic activities at CHOm1 and CHOm3 than A9Lm1 and A9Lm3 receptors. Although m2 receptor density was 6 fold higher in A9L than CHO cells, carbachol elicited significantly greater inhibition of adenosine 3':5'-cyclic monophosphate (cyclic AMP) formation in CHOm2 cells. These data suggest that not only receptor density but receptor-effector coupling and/or coupling efficiencies play significant roles in agonist-induced responses. 3. In CHO cells, receptor-effector coupling efficiencies were m3 = m1 > m5. Although CHOm5 receptors were the least efficiently coupled, some partial agonists displayed higher intrinsic efficacies at m5 than m3 receptors suggesting that, in CHO cells, m5 and m3 receptors may activate different G proteins and/or effectors to stimulate inositol monophosphate (IP1) formation. 4. McN-A-343 was a functionally selective m4 agonist. It had little or no agonist activity at m3 receptors expressed in either A9L or CHO cells.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atack J. R., Prior A. M., Griffith D., Ragan C. I. Characterization of the effects of lithium on phosphatidylinositol (PI) cycle activity in human muscarinic m1 receptor-transfected CHO cells. Br J Pharmacol. 1993 Oct;110(2):809–815. doi: 10.1111/j.1476-5381.1993.tb13884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgold J., White T. Pharmacological differences between muscarinic receptors coupled to phosphoinositide turnover and those coupled to adenylate cyclase inhibition. Biochem Pharmacol. 1989 May 15;38(10):1605–1616. doi: 10.1016/0006-2952(89)90308-0. [DOI] [PubMed] [Google Scholar]
- Birdsall N. J., Burgen A. S., Hulme E. C., Stockton J. M., Zigmond M. J. The effect of McN-A-343 on muscarinic receptors in the cerebral cortex and heart. Br J Pharmacol. 1983 Feb;78(2):257–259. doi: 10.1111/j.1476-5381.1983.tb09388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Kendall D. A., Nahorski S. R. Inositol phospholipid hydrolysis in rat cerebral cortical slices: I. Receptor characterisation. J Neurochem. 1984 May;42(5):1379–1387. doi: 10.1111/j.1471-4159.1984.tb02798.x. [DOI] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Buckley C. M., Brann M. R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989 Apr;35(4):469–476. [PubMed] [Google Scholar]
- Caulfield M. P. Muscarinic receptors--characterization, coupling and function. Pharmacol Ther. 1993 Jun;58(3):319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dokas L. A., Ting S. M. A comparison of the regulatory properties of striatal and cortical adenylate cyclase. Neurobiol Aging. 1993 Jan-Feb;14(1):65–72. doi: 10.1016/0197-4580(93)90024-6. [DOI] [PubMed] [Google Scholar]
- Fleming W. W., Westfall D. P., De la Lande I. S., Jellett L. B. Log-normal distribution of equiefective doses of norepinephrine and acetylcholine in several tissues. J Pharmacol Exp Ther. 1972 May;181(2):339–345. [PubMed] [Google Scholar]
- Hu J. R., el-Fakahany E. E. Selectivity of McN-A-343 in stimulating phosphoinositide hydrolysis mediated by M1 muscarinic receptors. Mol Pharmacol. 1990 Dec;38(6):895–903. [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Keen M., Nahorski S. R. Muscarinic acetylcholine receptors linked to the inhibition of adenylate cyclase activity in membranes from the rat striatum and myocardium can be distinguished on the basis of agonist efficacy. Mol Pharmacol. 1988 Dec;34(6):769–778. [PubMed] [Google Scholar]
- Kenakin T. P., Boselli C. Promiscuous or heterogeneous muscarinic receptors in rat atria? I. Schild analysis with simple competitive antagonists. Eur J Pharmacol. 1990 Nov 20;191(1):39–48. doi: 10.1016/0014-2999(90)94094-e. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazareno S., Farries T., Birdsall N. J. Pharmacological characterization of guanine nucleotide exchange reactions in membranes from CHO cells stably transfected with human muscarinic receptors m1-m4. Life Sci. 1993;52(5-6):449–456. doi: 10.1016/0024-3205(93)90301-i. [DOI] [PubMed] [Google Scholar]
- Li M., Yasuda R. P., Wall S. J., Wellstein A., Wolfe B. B. Distribution of m2 muscarinic receptors in rat brain using antisera selective for m2 receptors. Mol Pharmacol. 1991 Jul;40(1):28–35. [PubMed] [Google Scholar]
- McKinney M., Anderson D., Vella-Rountree L. Different agonist-receptor active conformations for rat brain M1 and M2 muscarinic receptors that are separately coupled to two biochemical effector systems. Mol Pharmacol. 1989 Jan;35(1):39–47. [PubMed] [Google Scholar]
- McKinney M., Miller J. H., Gibson V. A., Nickelson L., Aksoy S. Interactions of agonists with M2 and M4 muscarinic receptor subtypes mediating cyclic AMP inhibition. Mol Pharmacol. 1991 Dec;40(6):1014–1022. [PubMed] [Google Scholar]
- Mitchelson F. Muscarinic receptor differentiation. Pharmacol Ther. 1988;37(3):357–423. doi: 10.1016/0163-7258(88)90005-8. [DOI] [PubMed] [Google Scholar]
- Nilvebrant L., Sparf B. Dicyclomine, benzhexol and oxybutynine distinguish between subclasses of muscarinic binding sites. Eur J Pharmacol. 1986 Apr 9;123(1):133–143. doi: 10.1016/0014-2999(86)90697-7. [DOI] [PubMed] [Google Scholar]
- Olianas M. C., Onali P. Muscarinic stimulation of adenylate cyclase activity of rat olfactory bulb. II. Characterization of the antagonist sensitivity and comparison with muscarinic inhibitions of the enzyme in striatum and heart. J Pharmacol Exp Ther. 1991 Nov;259(2):680–686. [PubMed] [Google Scholar]
- Richards M. H. Pharmacology and second messenger interactions of cloned muscarinic receptors. Biochem Pharmacol. 1991 Oct 9;42(9):1645–1653. doi: 10.1016/0006-2952(91)90498-t. [DOI] [PubMed] [Google Scholar]
- Richards M. H. Rat hippocampal muscarinic autoreceptors are similar to the M2 (cardiac) subtype: comparison with hippocampal M1, atrial M2 and ileal M3 receptors. Br J Pharmacol. 1990 Apr;99(4):753–761. doi: 10.1111/j.1476-5381.1990.tb13002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. D., Davis R. E., Jaen J. C., Spencer C. J., Tecle H., Thomas A. J. Characterization of muscarinic agonists in recombinant cell lines. Life Sci. 1993;52(5-6):465–472. doi: 10.1016/0024-3205(93)90303-k. [DOI] [PubMed] [Google Scholar]
- Varrault A., Journot L., Audigier Y., Bockaert J. Transfection of human 5-hydroxytryptamine1A receptors in NIH-3T3 fibroblasts: effects of increasing receptor density on the coupling of 5-hydroxytryptamine1A receptors to adenylyl cyclase. Mol Pharmacol. 1992 Jun;41(6):999–1007. [PubMed] [Google Scholar]
- Wall S. J., Yasuda R. P., Hory F., Flagg S., Martin B. M., Ginns E. I., Wolfe B. B. Production of antisera selective for m1 muscarinic receptors using fusion proteins: distribution of m1 receptors in rat brain. Mol Pharmacol. 1991 May;39(5):643–649. [PubMed] [Google Scholar]
- Wang S. Z., el-Fakahany E. E. Application of transfected cell lines in studies of functional receptor subtype selectivity of muscarinic agonists. J Pharmacol Exp Ther. 1993 Jul;266(1):237–243. [PubMed] [Google Scholar]
- Wojcikiewicz R. J., Tobin A. B., Nahorski S. R. Desensitization of cell signalling mediated by phosphoinositidase C. Trends Pharmacol Sci. 1993 Jul;14(7):279–285. doi: 10.1016/0165-6147(93)90131-3. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]
- Worton R. G. Karyotypic heterogeneity in CHO cell lines. Cytogenet Cell Genet. 1978;21(1-2):105–110. doi: 10.1159/000130883. [DOI] [PubMed] [Google Scholar]
- Yasuda R. P., Ciesla W., Flores L. R., Wall S. J., Li M., Satkus S. A., Weisstein J. S., Spagnola B. V., Wolfe B. B. Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: distribution of m4 and m5 receptors in rat brain. Mol Pharmacol. 1993 Feb;43(2):149–157. [PubMed] [Google Scholar]


