Abstract
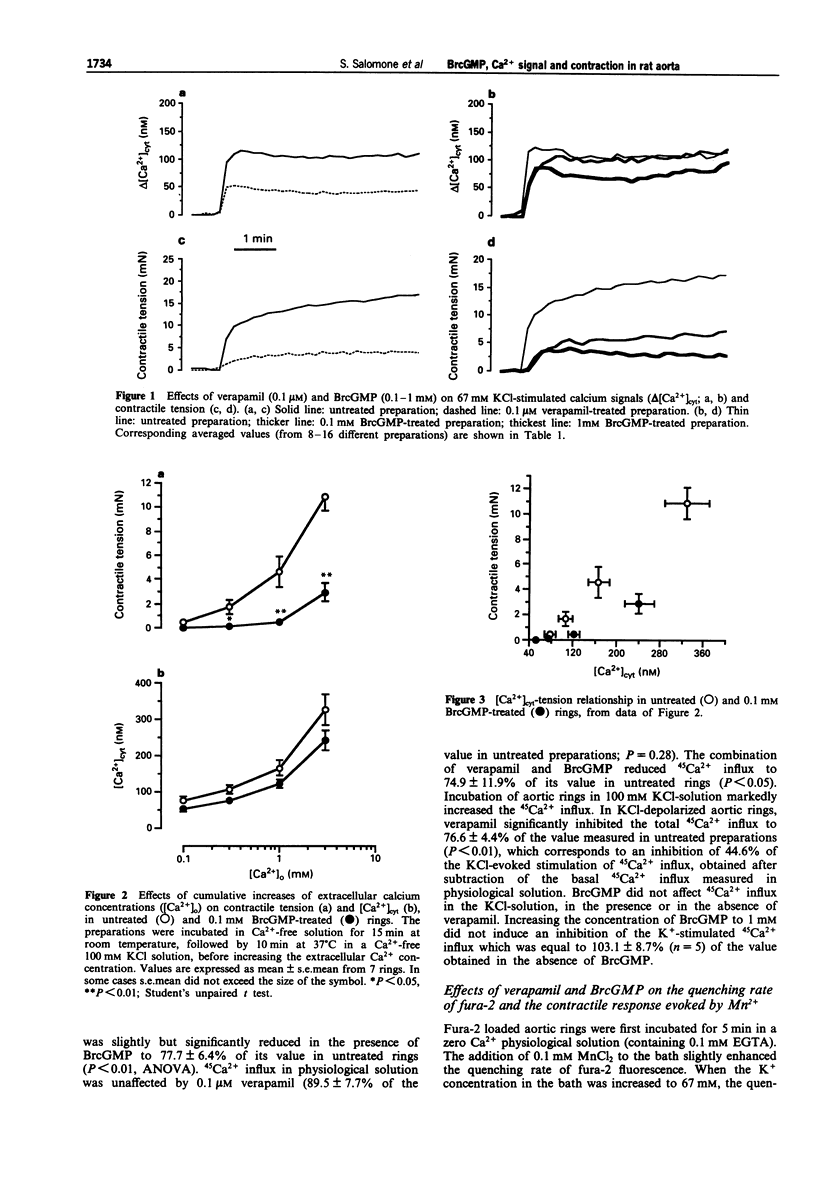
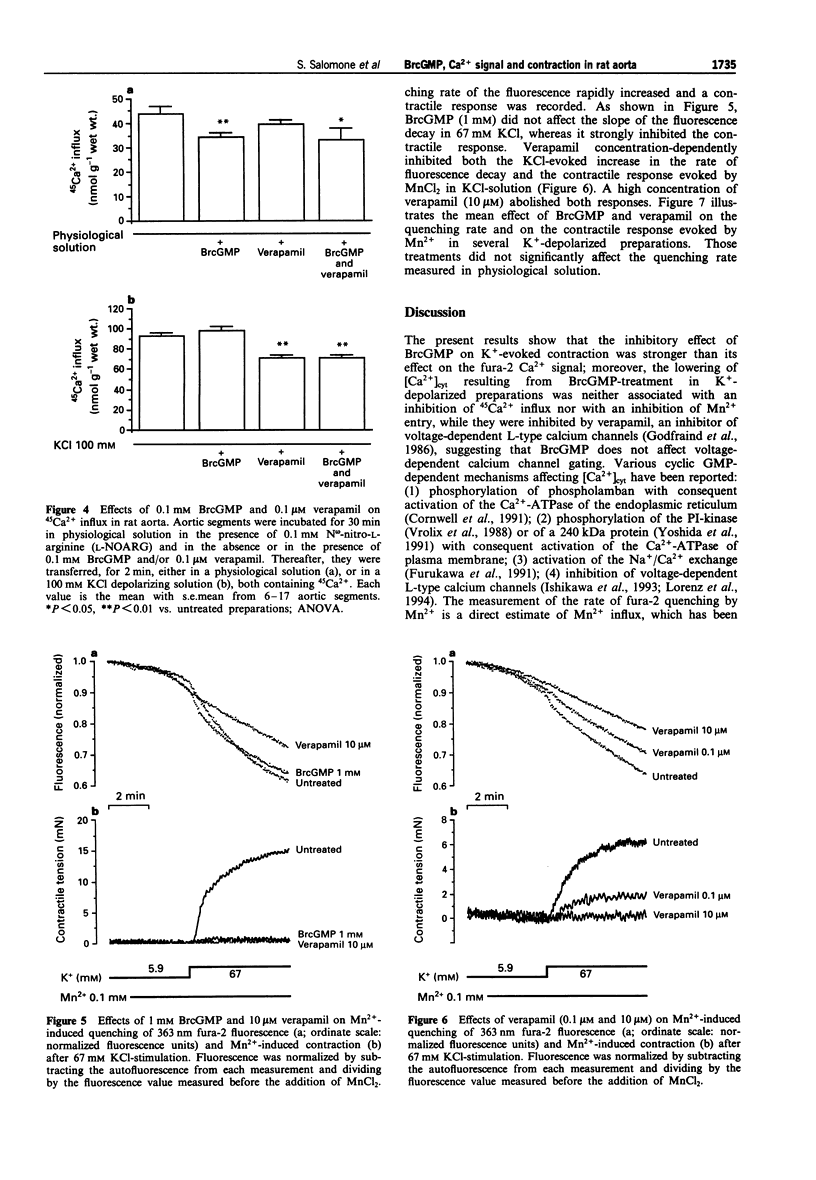
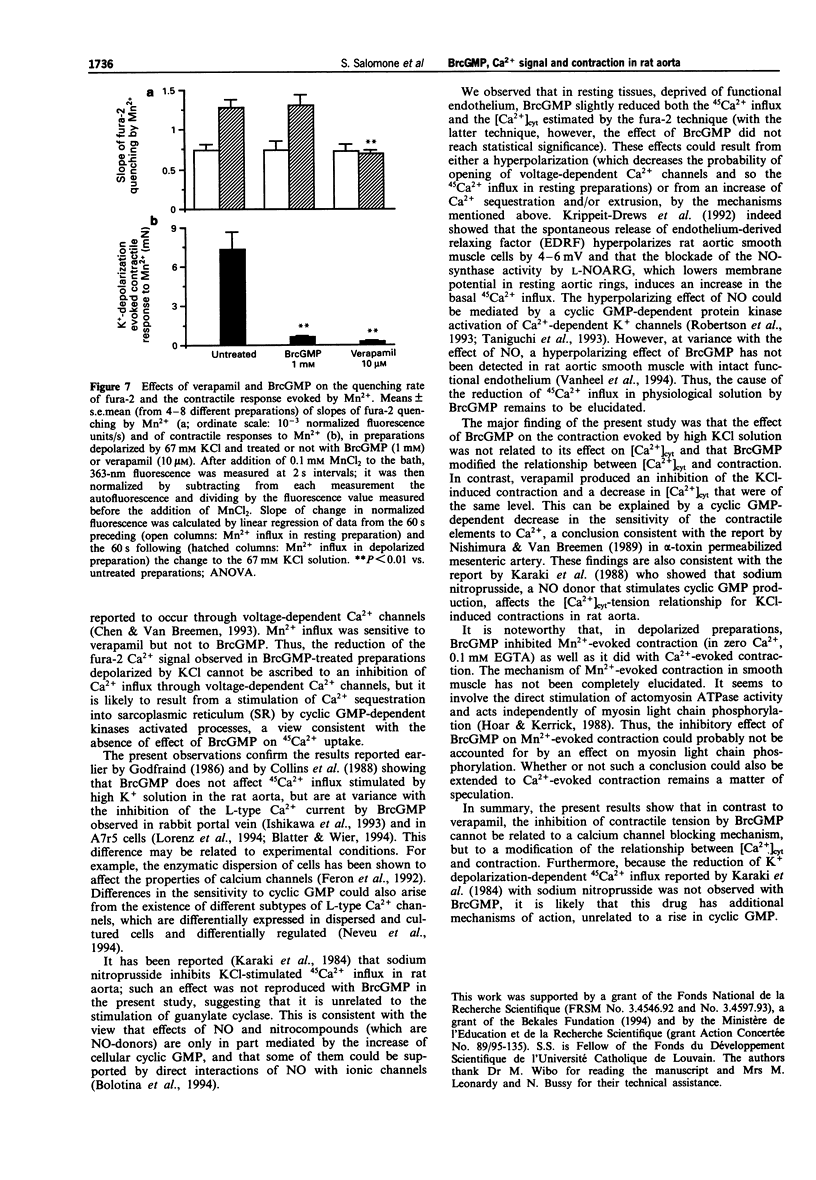
1. The pharmacological action of NO donors is usually attributed to a cellular rise in guanosine 3':5'-cyclic monophosphate (cyclic GMP), but this hypothesis is based only on indirect evidence. Therefore, we have studied the effects of cyclic GMP on Ca2+ movements and contraction in rat isolated endothelium-denuded aorta stimulated by KCl depolarizing solution using the permeant analogue 8-bromo cyclic GMP (BrcGMP). Isometric contraction and fura-2 Ca2+ signals were measured simultaneously in preparations treated with BrcGMP and with verapamil. The activation of calcium channels was estimated by measuring the quenching rate of the intracellular fura-2 signal by Mn2+ and by the depolarization-dependent influx of 45Ca2+. 2. Stimulation with 67 mM KCl-solution evoked an increase in cytosolic Ca2+ concentration ([Ca2+]cyt) and a contractile response which were inhibited by pretreatment with verapamil (0.1 microM) or BrcGMP (0.1-1 mM). However, the inhibition of the fura-2 Ca2+ signal was significantly higher with verapamil than with BrcGMP, whereas the contraction was inhibited to a similar extent. 3. When preparations were exposed to K(+)-depolarizing solution in which the calcium concentration was cumulatively increased, the related increase in fura-2 Ca2+ signal was barely affected by BrcGMP, whereas the contractile tension was strongly and significantly inhibited. 4. Cellular Ca2+ changes were also estimated with 45Ca2+. 45Ca2+ influx in resting preparations was significantly reduced by BrcGMP (0.1 mM) but not by verapamil (0.1 microM); 45Ca2+ influx in KCl-depolarized preparations was reduced by verapamil but was unaffected by BrcGMP.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatter L. A., Wier W. G. Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current. Cell Calcium. 1994 Feb;15(2):122–131. doi: 10.1016/0143-4160(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Bolotina V. M., Najibi S., Palacino J. J., Pagano P. J., Cohen R. A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994 Apr 28;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Braughler J. M., Mittal C. K., Murad F. Effects of thiols, sugars, and proteins on nitric oxide activation of guanylate cyclase. J Biol Chem. 1979 Dec 25;254(24):12450–12454. [PubMed] [Google Scholar]
- Chen Q., van Breemen C. The superficial buffer barrier in venous smooth muscle: sarcoplasmic reticulum refilling and unloading. Br J Pharmacol. 1993 Jun;109(2):336–343. doi: 10.1111/j.1476-5381.1993.tb13575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Henderson A. H., Lang D., Lewis M. J. Endothelium-derived relaxing factor and nitroprusside compared in noradrenaline- and K+-contracted rabbit and rat aortae. J Physiol. 1988 Jun;400:395–404. doi: 10.1113/jphysiol.1988.sp017127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell T. L., Pryzwansky K. B., Wyatt T. A., Lincoln T. M. Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol Pharmacol. 1991 Dec;40(6):923–931. [PubMed] [Google Scholar]
- Dietrich H. H., Kimura M., Dacey R. G., Jr N omega-nitro-L-arginine constricts cerebral arterioles without increasing intracellular calcium levels. Am J Physiol. 1994 Apr;266(4 Pt 2):H1681–H1686. doi: 10.1152/ajpheart.1994.266.4.H1681. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Kelm M. Biotransformation of organic nitrates to nitric oxide by vascular smooth muscle and endothelial cells. Biochem Biophys Res Commun. 1991 Oct 15;180(1):286–293. doi: 10.1016/s0006-291x(05)81290-2. [DOI] [PubMed] [Google Scholar]
- Feron O., Wibo M., Christen M. O., Godfraind T. Interaction of pinaverium (a quaternary ammonium compound) with 1,4-dihydropyridine binding sites in rat ileum smooth muscle. Br J Pharmacol. 1992 Feb;105(2):480–484. doi: 10.1111/j.1476-5381.1992.tb14279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Ohshima N., Tawada-Iwata Y., Shigekawa M. Cyclic GMP stimulates Na+/Ca2+ exchange in vascular smooth muscle cells in primary culture. J Biol Chem. 1991 Jul 5;266(19):12337–12341. [PubMed] [Google Scholar]
- Godfraind T. Calcium exchange in vascular smooth muscle, action of noradrenaline and lanthanum. J Physiol. 1976 Aug;260(1):21–35. doi: 10.1113/jphysiol.1976.sp011501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T. EDRF and cyclic GMP control gating of receptor-operated calcium channels in vascular smooth muscle. Eur J Pharmacol. 1986 Jul 31;126(3):341–343. doi: 10.1016/0014-2999(86)90070-1. [DOI] [PubMed] [Google Scholar]
- Godfraind T., Miller R., Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986 Dec;38(4):321–416. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gupta S., McArthur C., Grady C., Ruderman N. B. Stimulation of vascular Na(+)-K(+)-ATPase activity by nitric oxide: a cGMP-independent effect. Am J Physiol. 1994 May;266(5 Pt 2):H2146–H2151. doi: 10.1152/ajpheart.1994.266.5.H2146. [DOI] [PubMed] [Google Scholar]
- Hoar P. E., Kerrick W. G. Mn2+ activates skinned smooth muscle cells in the absence of myosin light chain phosphorylation. Pflugers Arch. 1988 Aug;412(3):225–230. doi: 10.1007/BF00582501. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Hume J. R., Keef K. D. Regulation of Ca2+ channels by cAMP and cGMP in vascular smooth muscle cells. Circ Res. 1993 Dec;73(6):1128–1137. doi: 10.1161/01.res.73.6.1128. [DOI] [PubMed] [Google Scholar]
- Karaki H., Nakagawa H., Urakawa N. Comparative effects of verapamil and sodium nitroprusside on contraction and 45Ca uptake in the smooth muscle of rabbit aorta, rat aorta and guinea-pig taenia coli. Br J Pharmacol. 1984 Feb;81(2):393–400. doi: 10.1111/j.1476-5381.1984.tb10091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H., Sato K., Ozaki H., Murakami K. Effects of sodium nitroprusside on cytosolic calcium level in vascular smooth muscle. Eur J Pharmacol. 1988 Nov 1;156(2):259–266. doi: 10.1016/0014-2999(88)90329-9. [DOI] [PubMed] [Google Scholar]
- Krippeit-Drews P., Morel N., Godfraind T. Effect of nitric oxide on membrane potential and contraction of rat aorta. J Cardiovasc Pharmacol. 1992;20 (Suppl 12):S72–S75. doi: 10.1097/00005344-199204002-00022. [DOI] [PubMed] [Google Scholar]
- Lorenz J. N., Bielefeld D. R., Sperelakis N. Regulation of calcium channel current in A7r5 vascular smooth muscle cells by cyclic nucleotides. Am J Physiol. 1994 Jun;266(6 Pt 1):C1656–C1663. doi: 10.1152/ajpcell.1994.266.6.C1656. [DOI] [PubMed] [Google Scholar]
- McDaniel N. L., Chen X. L., Singer H. A., Murphy R. A., Rembold C. M. Nitrovasodilators relax arterial smooth muscle by decreasing [Ca2+]i and uncoupling stress from myosin phosphorylation. Am J Physiol. 1992 Aug;263(2 Pt 1):C461–C467. doi: 10.1152/ajpcell.1992.263.2.C461. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Neveu D., Quignard J. F., Fernandez A., Richard S., Nargeot J. Differential beta-adrenergic regulation and phenotypic modulation of voltage-gated calcium currents in rat aortic myocytes. J Physiol. 1994 Sep 1;479(Pt 2):171–182. doi: 10.1113/jphysiol.1994.sp020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989 Sep 15;163(2):929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pfitzer G., Merkel L., Rüegg J. C., Hofmann F. Cyclic GMP-dependent protein kinase relaxes skinned fibers from guinea pig taenia coli but not from chicken gizzard. Pflugers Arch. 1986 Jul;407(1):87–91. doi: 10.1007/BF00580726. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Robertson B. E., Schubert R., Hescheler J., Nelson M. T. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993 Jul;265(1 Pt 1):C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Lohmann S. M., Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993 Aug 18;1178(2):153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Sekhar K. R., Hatchett R. J., Shabb J. B., Wolfe L., Francis S. H., Wells J. N., Jastorff B., Butt E., Chakinala M. M., Corbin J. D. Relaxation of pig coronary arteries by new and potent cGMP analogs that selectively activate type I alpha, compared with type I beta, cGMP-dependent protein kinase. Mol Pharmacol. 1992 Jul;42(1):103–108. [PubMed] [Google Scholar]
- Taniguchi J., Furukawa K. I., Shigekawa M. Maxi K+ channels are stimulated by cyclic guanosine monophosphate-dependent protein kinase in canine coronary artery smooth muscle cells. Pflugers Arch. 1993 May;423(3-4):167–172. doi: 10.1007/BF00374390. [DOI] [PubMed] [Google Scholar]
- Vanheel B., Van de Voorde J., Leusen I. Contribution of nitric oxide to the endothelium-dependent hyperpolarization in rat aorta. J Physiol. 1994 Mar 1;475(2):277–284. doi: 10.1113/jphysiol.1994.sp020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrolix M., Raeymaekers L., Wuytack F., Hofmann F., Casteels R. Cyclic GMP-dependent protein kinase stimulates the plasmalemmal Ca2+ pump of smooth muscle via phosphorylation of phosphatidylinositol. Biochem J. 1988 Nov 1;255(3):855–863. doi: 10.1042/bj2550855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Sun H. T., Cai J. Q., Imai S. Cyclic GMP-dependent protein kinase stimulates the plasma membrane Ca2+ pump ATPase of vascular smooth muscle via phosphorylation of a 240-kDa protein. J Biol Chem. 1991 Oct 15;266(29):19819–19825. [PubMed] [Google Scholar]


