Abstract
Recombinant baculoviruses can serve as gene-transfer vehicles for transient expression of recombinant proteins in a wide range of mammalian cell types. Furthermore, by inclusion of a dominant selectable marker in the viral vector, cell lines can be derived that stably express recombinant genes. A virus was constructed containing two expression cassettes controlled by constitutive mammalian promoters: the cytomegalovirus immediate early promoter/enhancer directing expression of green fluorescent protein and the simian virus 40 (SV40) early promoter controlling neomycin phosphotransferase II. Using this virus, efficient gene delivery and expression was observed and measured in numerous cell types of human, primate, and rodent origin. In addition to commonly used transformed cell lines such as HeLa, CHO, Cos-7, and 293, this list includes primary human keratinocytes and bone marrow fibroblasts. In all cases, addition of butyrate or trichostatin A (a selective histone deacetylase inhibitor) to transduced cells markedly enhanced the levels of reporter protein expression observed. When transduced cells are put under selection with the antibiotic G418, cell lines can be obtained at high frequency that stably maintain the expression cassettes of the vector DNA and exhibit stable, high-level expression of the reporter gene. Stably transduced derivatives have been selected from a substantial number of different cell types, suggesting that stable lines can be derived from any cell type that exhibits transient expression.
The ability to efficiently transfer genes for expression in different cell types is central to the study of protein function. This has led to the development of several physical and chemical techniques to introduce foreign nucleic acids into cells. These methods are, to varying degrees, deleterious to cells and require large quantities of DNA. Viruses (by their nature) are efficient gene delivery vehicles. A common feature of most viral vector systems is that they are replication-deficient and require helper virus functions for propagation. Thus, the mechanism of viral entry to deliver foreign genes is exploited, whereas the harmful effects of a productive viral infection in recipient cells are avoided. Unfortunately, some replication-defective systems are cumbersome, requiring transfection steps or cloning of producer cell lines to generate virus stocks. Furthermore, the vector systems are susceptible to replication-competent virus breakthrough, raising biosafety concerns.
Although baculovirus expression vectors derived from the Autographa californica nuclear polyhedrosis virus have been used for many years to overexpress recombinant proteins in insect-derived host cells, investigators also have been interested in virus interactions with cell lines other than the host cells. Volkman and Goldsmith (1) demonstrated that virions were able to enter certain cell lines derived from vertebrate species, although no evidence of viral gene expression was detected. Subsequently, Carbonell and Miller (2), using a recombinant virus with a mammalian expression cassette, detected reporter-enzyme activity in a human lung carcinoma line. However, they concluded that the enzyme activity was not caused by de novo gene expression after viral entry into cells but was associated with the virion itself.
Several recent publications have demonstrated gene transfer and expression in mammalian cells mediated by recombinant baculoviruses carrying mammalian expression cassettes. Studies from two groups (3, 4) showed that primary human hepatocytes and cell lines of hepatic origin were able to efficiently take up and transiently express reporter genes under the control of constitutive mammalian promoters. These reports also looked at a panel of cell lines of nonhepatic origin and found only weak gene expression in isolated cases. Other workers have demonstrated an increased range of susceptible cell types for recombinant baculoviruses. By using a mammalian promoter in a baculovirus vector, expression of several gene products has been observed in human hepatoma cell lines, HeLa, Cos-7, and CPK (porcine kidney), as well as six other tumor cell lines from rat, porcine, and human sources (5, 6).
In this study, we describe the use of a recombinant baculovirus vector carrying a mammalian expression cassette comprising the cytomegalovirus immediate early (CMV-IE) promoter and the gene for green fluorescent protein (GFP) to direct gene expression in a wide variety of mammalian cell lines as well as primary human cells derived from different tissues. Expression of GFP in virus-treated cells is markedly increased by addition of butyrate or trichostatin A (TSA), a histone deacetylase inhibitor. Additionally, inclusion of an expression cassette encoding a dominant selectable marker in this vector allows for selection of cell lines that stably maintain expression of the reporter gene for multiple passages. These findings significantly expand the utility of baculoviruses as gene-transfer vectors for a broad spectrum of cell types.
MATERIALS AND METHODS
Generation of Recombinant Baculoviruses.
Viruses were constructed by using shuttle vectors derived from pFastBac1 (Life Technologies, Grand Island, NY). Plasmid DNA was digested with SnaBI and HpaI to remove the baculovirus polyhedrin gene promoter sequences. A 3.1-kbp NruI–Bst1107I fragment from pcDNA3 (Invitrogen), which contains the CMV-IE promoter/enhancer with a multiple cloning site and polyadenylation signal followed by the simian virus 40 (SV40) promoter-neomycin phosphotransferase II expression cassette, was inserted into the pFastBac1 backbone (pFastBacMam1). The shuttle plasmid pFastBac GFP contains the modified gene encoding GFP from pGreenLantern1 (Life Technologies) inserted into the NotI site of pFastBac1. The GFP gene was moved from pFastBac GFP to pFastBacMam1 as an EcoRI–XbaI fragment to construct pFastBacMam1 GFP (Fig. 1).
Figure 1.
Shuttle vector used to construct BacMam1 GFP baculovirus. A detailed description is in the text.
Recombinant virus (BacMam1 GFP) was generated by using the Bac-to-Bac system (Life Technologies). Virus was further amplified by propagation in Sf9 (Spodoptera frugiperda) cells grown in suspension in Grace’s supplemented insect media containing 10% (vol/vol) fetal bovine serum (HyClone), 0.1% (vol/vol) pluronic F-68, and 25 μg/ml gentamycin according to standard protocols (7). The CMV-IE promoter in this virus is utilized weakly in insect cells, resulting in expression of GFP in these cultures. Stocks of virus were concentrated by centrifugation at 35,000 × g for 60 min, and pelleted virus was resuspended in Dulbecco’s PBS supplemented with 1% (vol/vol) fetal bovine serum. Virus titers were determined by plaque assay on Sf9 cells.
Cell Culture.
The human hepatoma cell line HuH-7 (8) was obtained from W. Mason (Fox Chase Cancer Center, Philadelphia). T antigen CHO cells were obtained from E. Tate and J. Northrop (Affymax, Santa Clara, CA). The cell line W12, a HPV-16 immortalized human keratinocyte cell line (9), was obtained from P. Lambert (University of Wisconsin, Madison, WI). Primary hepatocytes were obtained from Clonetics (San Diego). Primary human keratinocytes were isolated from neonatal foreskins. Primary human bone marrow fibroblasts were prepared from bone marrow aspirates. All other cell lines listed in Table 1 were obtained from the American Type Culture Collection.
Table 1.
Transduction of mammalian cell lines with BacMam1 GFP baculovirus
| Cells | Percent transduced
|
Mean fluorescence, MESF × 10−6
|
||
|---|---|---|---|---|
| Virus | Virus + 10 mM butyrate | Virus | Virus + 10 mM butyrate | |
| HuH-7 | 91 ± 1.3 | 100 ± 0.2 | 1.2 ± 0.1 | 24.4 ± 1.5 |
| CHO | 72 ± 1.8 | 97 ± 0.7 | 1.1 ± 0.1 | 7.6 ± 0.6 |
| CHO-T Ag | 94 ± 2.5 | 99 ± 0.2 | 3.4 ± 0.3 | 16.2 ± 0.8 |
| CV-1 | 89 ± 1.7 | 95 ± 0.7 | 8.0 ± 0.4 | 46.8 ± 1.8 |
| CosV-7 | 86 ± 1.5 | 94 ± 1.2 | 8.9 ± 0.5 | 62.5 ± 4.1 |
| HeLa | 83 ± 2.6 | 98 ± 0.5 | 2.5 ± 0.3 | 56.6 ± 2.0 |
| 293 | 85 ± 2.8 | 98 ± 0.4 | 3.4 ± 0.3 | 15.9 ± 0.3 |
| BHK | 91 ± 1.2 | 99 ± 0.5 | 2.1 ± 0.06 | 52.9 ± 1.2 |
| WI38 | 84 ± 6.1 | 99 ± 0.3 | 12.3 ± 0.2 | 71.5 ± 4.1 |
| SK-N-MC | 90 ± 0.1 | 100 ± 0.1 | 1.4 ± 0.07 | 31.4 ± 1.2 |
| PC12 | 1 ± 0.3 | 19 ± 0.5 | 0.004 ± 0.003 | 0.34 ± 0.03 |
| Human keratinocytes | 61 ± 2.4 | 84 ± 1.7 | 1.7 ± 0.04 | 9.4 ± 0.8 |
| W12 | 89 ± 0.7 | 98 ± 0.5 | 19.2 ± 0.4 | 47.8 ± 0.7 |
| HBMF | 46 ± 2.9 | 84 ± 2.1 | 0.44 ± 0.04 | 6.6 ± 0.7 |
| MG63 | 92 ± 2.9 | 97 ± 0.7 | 2.6 ± 0.5 | 17.1 ± 3.4 |
| THP-1 (+PMA) | 7 ± 0.7 | 5 ± 0.8 | 0.007 ± 0.003 | 0.04 ± 0.008 |
| K562 | 1 ± 0.1 | 18 ± 1.2 | 0.007 ± 0.005 | 0.12 ± 0.004 |
| Raw264.7 | 2 ± 0.3 | 8 ± 0.5 | 0.007 ± 0.001 | 0.03 ± 0.008 |
| P388D1 | 2 ± 0.2 | 26 ± 5.3 | 0.002 ± 0.0004 | 0.4 ± 0.12 |
| U937 | 0 | 0 | 0 | 0 |
Results are averages of three independent transductions. Errors are standard deviations. MESF, molecules of equivalent soluble fluorochrome. PMA, phorbol 12-myristate 13-acetate.
Transduction of Mammalian Cells by BacMam1 GFP Virus.
Cells were seeded in 6-well culture dishes at 150,000–250,000 cells per well. Culture medium was removed, replaced with virus inoculum, and incubated for 1 hr at 37°C. After removal of virus, fresh medium was added and cultures were incubated at 37°C. Cells that grew in suspension were pelleted by centrifugation before addition of virus inoculum. In the case of THP-1 cells, phorbol 12-myristate 13-acetate was added to a concentration of 1 nM before transduction. Where indicated, culture media was supplemented with sodium butyrate or TSA (Shionogi, Osaka) immediately after virus treatment. Cultures were examined for GFP expression by using fluorescence microscopy. For Western blot analysis, cell extracts were resolved in denaturing polyacrylamide gels, and proteins were transferred to nitrocellulose membranes and immunoblotted by using standard methods (10). For selection of stable derivatives of transduced cells, culture media was supplemented with 500 μg/ml Geneticin (G418, Life Technologies).
Flow Cytometry.
Cultures were harvested with trypsin 24 hr posttransduction and washed and resuspended in Dulbecco’s PBS with 1% (vol/vol) fetal bovine serum. Data collection was performed on a Becton Dickinson FACStarplus flow cytometer. To correlate data taken during different analytical runs, Quantum GFP particles (Flow Cytometry Standards, San Juan, Puerto Rico) were analyzed to determine a standard curve of molecules of equivalent soluble fluorochrome (MESF) for GFP, which was applied to sample data from corresponding experiments. Sample data, converted to MESF values, could be compared across various experiments. When fluorescence signals were obtained beyond the fourth decade of intensity, a neutral-density filter (Oriel, Stamford, CT) was inserted in the light path of the detector to reduce the sample intensity to measurable values.
Electron Microscopy.
Chinese hamster ovary (CHO) cells were incubated with BacMam1 GFP virus for 1 hr at 37°C. Cells were washed, fixed in 2.5% glutaraldehyde and 0.1 M sodium cacodylate (pH 7.3), and embedded in Spurr resin (Electron Microscopy Science, Ft. Washington, PA). Ultrathin sections were cut, placed on copper grids, and stained with uranyl acetate. Grids were examined on a JEOL 100C transmission electron microscope.
Southern Blot Analysis.
Cellular DNA was prepared by using Qiagen (Chatsworth, CA) purification reagents and protocols. Baculoviral DNA was prepared from high-titer stocks of BacMam1 GFP virus by using standard methods (7). DNA samples were digested with StuI and electrophoresed on 0.8% agarose gels and transferred to Biodyne B nylon membranes (Pall) by using standard methods (11). Radioactive probe was generated by nick-translation of pFastBacMam1 GFP DNA (Nick Translation System; Life Technologies), and autoradiograms were developed by exposure of membranes to x-ray film.
RESULTS
Baculovirus-Mediated Gene Delivery to CHO Cells.
A recombinant baculovirus, BacMam1 GFP, which contains two mammalian expression cassettes, was constructed as described in Materials and Methods. By using this virus, efficient GFP expression was observed in both primary human hepatocytes and HepG2 human hepatoma cells (data not shown) as previously reported (3, 4). While investigating the ability to transduce other cell lines, we found that CHO cells also were susceptible to baculovirus-mediated gene transfer. GFP expression was observed in CHO cells 24 hr posttreatment with BacMam1 GFP at a multiplicity of infection (moi) of 200 plaque forming units (pfu) per cell (Fig. 2A). Expression of GFP could be visually detected as early as 4–6 hr after virus treatment. As seen in Fig. 2A, individual cells display a wide range of GFP expression. This visual result is represented in the histogram of cell suspensions analyzed by using flow cytometry (Fig. 2C, blue curve); the range of fluorescence intensities observed in the population is spread over approximately three orders of magnitude.
Figure 2.
Transduction of CHO cells with BacMam1 GFP baculovirus. (A and B) Visualization of GFP-expressing cells by fluorescence microscopy. (A) Cells transduced with BacMam1 GFP. (B) Same as A except for addition of 1 mM butyrate after incubation with virus. (C) Analysis of GFP expression by flow cytometry. Black line, nontransduced cells; blue line, transduced cells; red line, transduced cells plus 1 mM butyrate; green line, same as red line but with neutral-density filter in place.
Sodium butyrate has been demonstrated to affect transcriptional regulation and enhance gene expression in eukaryotic cells (12); thus, we investigated its effect on expression from the mammalian promoters in the baculovirus vector. Sodium butyrate was added to CHO cell culture medium at a concentration of 1 mM after incubation of cells with BacMam1 GFP. Cultures were examined 24 hr later by using fluorescence microscopy (Fig. 2B). As compared with the untreated control (Fig. 2A), it is apparent that butyrate has a significant effect on GFP expression, as essentially 100% of the cells exhibit an intense green color. In the histograms in Fig. 2C; the curve of the cell population treated with butyrate is shifted off scale (red curve) and a neutral-density filter with a reduction factor of 10 was required to put the curve on scale with the other samples (green curve). These results are mirrored in Western blots of BacMam1 GFP transduced cell extracts (Fig. 3) probed with antibodies directed against either GFP or NPT II. These results indicate that butyrate significantly enhances expression of genes controlled by either the CMV-IE promoter/enhancer or the SV40 promoter.
Figure 3.
Western blot analysis of GFP and NPT II expression in CHO cells. Extracts of cells were prepared 24 hr posttransduction. Lanes 1–3, cells probed with antibody directed against GFP; lanes 4–6, probed with antibody directed against NPT II. Lanes 1 and 4, nontransduced cells; lanes 2 and 5, transduced cells; lanes 3 and 6, transduced cells plus 1 mM butyrate. KDa, kilodaltons.
A sample of BacMam1 GFP was banded in a sucrose gradient. Fractions from the gradient that lead to a peak of GFP expression in insect cell cultures are coincident with the fractions that give rise to maximal GFP expression in CHO cell cultures (data not shown). This strongly suggests that GFP expression seen in the mammalian cells is mediated by the baculovirus construct.
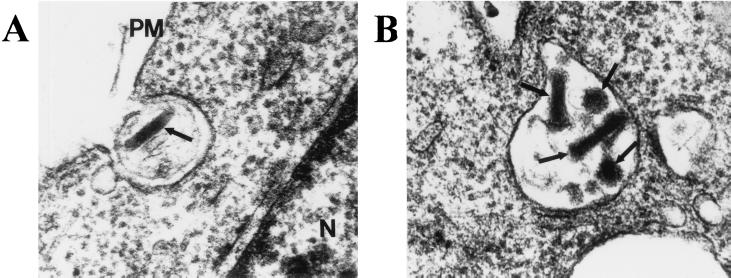
Cultures of CHO cells treated with BacMam1 GFP virus were examined by using electron microscopy to visualize virions associated with the cells. Fig. 4A shows a CHO cell apparently in the process of taking up a baculovirus particle by endocytosis. This mode of virus entry is consistent with published reports of entry of budded baculoviruses into target insect cells (13, 14), and also is consistent with the inhibition of gene transfer in hepatoma cells by chloriquine (3, 4). Many cells in the sample contained one or more virions in the cytoplasm of the cell; some virions also were observed in vesicles (Fig. 4B). This result is similar to the findings of Hofmann et al. (3) in HuH-7 cells incubated with a baculovirus vector.
Figure 4.
Detection of baculovirus particles in CHO cells by electron microscopy. (A) Virus particle at the surface of cell. (×85,000.) (B) Virus particles enclosed in membrane vesicle. (×73,000.) Arrows denote virus particles. PM, plasma membrane; N, nucleus.
Range of Susceptibility of Mammalian Cell Types to Baculovirus Transduction.
The results described above indicate that the baculovirus vector is efficiently internalized by CHO cells, consistent with other reports that demonstrated delivery to cells other than those of hepatic origin (5, 6). A further investigation of the range of cell types susceptible to transduction with BacMam1 GFP is summarized in Table 1. Twenty different cell types were transduced at a moi of 200 pfu per cell carried out in the absence or presence of 10 mM sodium butyrate and subsequently analyzed by flow cytometry. This analysis allows examination of individual cells for GFP expression. All samples were examined together with a fluorescent standard so that relative fluorescence values could be reported to estimate the fold enhancement of expression achieved by addition of butyrate, and the level of GFP expression could be compared between individual cell lines.
The data in Table 1 show that the baculovirus vector is able to transduce a wide variety of cell types originating from different tissues. Several commonly used cell lines such as HuH-7, CHO, CV-1, Cos-7, HeLa, and 293 cells are all efficiently transduced by BacMam1 GFP at frequencies ranging from 72% to 94%. Different cell lines exhibit different levels of GFP expression, as evidenced by their different mean fluorescence values. This may reflect differing regulation of the CMV-IE promoter in the cell types examined. In all cases, addition of 10 mM butyrate to the cultures after transduction resulted in elevated levels of GFP expression. In some instances (e.g., HeLa, BHK) enhancement of expression from the CMV-IE promoter/enhancer is quite dramatic, reaching 20-fold higher mean fluorescence values. Of all cell types examined in this study, W12 and WI38 cells exhibit the highest levels of GFP expression, although we observed only a 2.5- to 6-fold enhancement with 10 mM butyrate.
The mammalian expression cassette portion of the BacMam1 GFP recombinant baculovirus contains an SV40 origin of replication. Transfection of a plasmid containing this DNA sequence into cells that express SV40 large T antigen allows for amplification of the DNA and increased expression of gene products encoded by the plasmid (15). However, the efficiency of transduction and level of GFP expression does not appear to be significantly influenced after transduction of cells that constitutively produce T antigen (e.g., CHO vs. CHO-T antigen and CV-1 vs. Cos-7).
Several cell types listed in Table 1 are transduced to a lesser extent than those mentioned above. Addition of butyrate to cultures enhances expression of the reporter gene, thus increasing the apparent transduction frequency in all but one cell line (THP-1). This is most strikingly illustrated in the results exhibited by the cell lines PC12, K562, and P388D1. Only 1–2% of the cells in these cultures are transduced when treated with virus alone; however, in cultures incubated with 10 mM butyrate after virus treatment, approximately 20% of the cells show detectable GFP expression. Additionally, these cell lines demonstrate some of the most remarkable increases in expression resulting from butyrate treatment; GFP expression increases 85-fold or greater in PC12 and P388D1 cells. The U937 hemopoietic cell line is the only one tested that was not transduced by the baculovirus vector. However, these cells were tested only under one set of conditions, and it may be possible that culture conditions exist in which U937 cells are transducable.
Cells derived from a wide variety of tissue types are included in Table 1 to illustrate the broad range of utility that baculovirus vectors containing mammalian expression cassettes can have for gene expression studies. All of the cell lines derived from kidney tissue (BHK, CV-1, Cos-7, and 293 cells) are transduced quite efficiently. The neuroblastoma line, SK-N-MC is efficiently transduced, whereas another cell line of neuronal origin, the rat pheochromocytoma line PC12, is transduced at a low level. Primary human keratinocytes and primary human bone marrow fibroblasts (HBMF in Table 1) along with a HPV-16 immortalized keratinocyte cell line, W12, and an osteosarcoma cell line (MG63) also are susceptible to baculovirus-mediated transduction. Finally, we also have observed transient baculovirus-mediated GFP expression in Caco-2 intestinal carcinoma cells, BeWo chondrocytoma cells, primary adipocytes from rat, primary human bladder smooth muscle cells, and frog melanophores (data not shown).
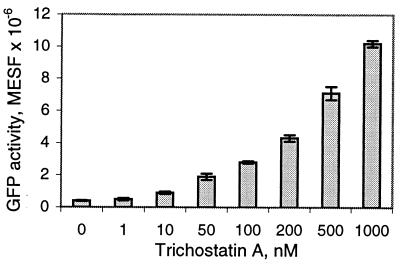
In all cells shown in Table 1, addition of sodium butyrate enhances expression levels of the reporter. Although butyrate has a variety of effects on different cell types, the underlying mechanism behind the ability of butyrate to stimulate transcription of certain genes is thought to be mediated by inhibition of the enzyme histone deacetylase (12). TSA is a specific, potent inhibitor of histone deacetylase (16); therefore, we examined the effect of TSA on BacMam1 GFP-transduced CHO cells. A dose response of GFP expression at increasing concentrations of TSA is shown in Fig. 5. Levels of GFP expression increase with increasing TSA through two orders of magnitude of concentration, from 10 nM to 1 μM. As mentioned above, TSA is more potent than butyrate, yielding a similar enhancement of GFP expression at 1 μM as is observed at 10 mM butyrate. These data suggest that the CMV-IE promoter/enhancer-controlled expression cassette delivered by the recombinant baculovirus is directly or indirectly susceptible to silencing by histones, which can be alleviated by histone hyperacetylation.
Figure 5.
Effect of TSA on GFP expression in CHO cells transduced with BacMam1 GFP baculovirus. Transduced cells were incubated for 24 hr in the presence of the indicated concentrations of TSA and GFP quantified by using flow cytometry. Results are the average of three independent transductions.
Stable Expression Derived from Baculovirus Transduced Cells.
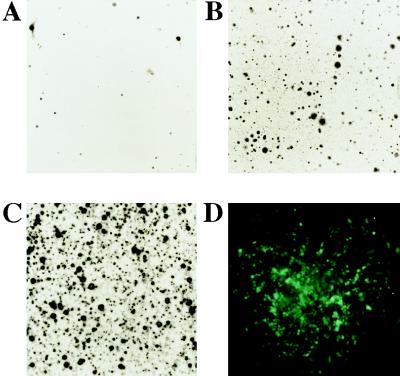
Results shown above demonstrate that baculovirus vectors are able to deliver DNA into mammalian cells in a form recognizable by the transcription machinery for expressing recombinant genes. Furthermore, no obvious toxic effects were detected by using microscopic examination of transduced cells. Thus, we reasoned that including an expression cassette encoding a dominant-selectable marker in the virus might allow the selection of stably transduced cells. This would be similar to hepatitis B virus where, although the viral DNA does not integrate into the host genome as part of its life cycle, integration events occur that lead to cells stably expressing viral gene products (17). BacMam1 GFP contains an expression cassette that allows selection of cells in the presence of the antibiotic G418. CHO cells were treated with virus at a moi of 1, 10, and 100 pfu per cell and incubated in media supplemented with G418 at 500 μg/ml for 14 days, at which time the cultures were stained with crystal violet (Fig. 6 A–C). Colonies of cells developed in the selective media in a dose-dependent manner with respect to virus inoculum.
Figure 6.
Formation of stably transduced CHO cells with BacMam1 GFP. (A–C) Effect of virus inoculum on number of G418-resistant clones. Cells were transduced with virus at different moi and clones were selected as described in the text. (A) moi of 1. (B) moi of 10. (C) moi of 100. (D) Fluorescence micrograph of a stably transduced clone.
To investigate the stability of GFP expression, transduced CHO cells were placed in selective media until colonies of G418-resistant cells were visible. As visualized by fluorescence microscopy, colonies exhibited a distribution of fluorescence levels from intensely green (representing high GFP expression) to no detectable fluorescence (data not shown). Colonies were not obtained from cultures that had not received virus. Resistant colonies were pooled, and clonal lines were obtained by cell sorting those that produced GFP. A representative colony from a clonal line that exhibits a high level of GFP expression is shown in Fig. 6D. Four randomly chosen clones maintained GFP expression after culture for at least 25 passages in G418-supplemented medium.
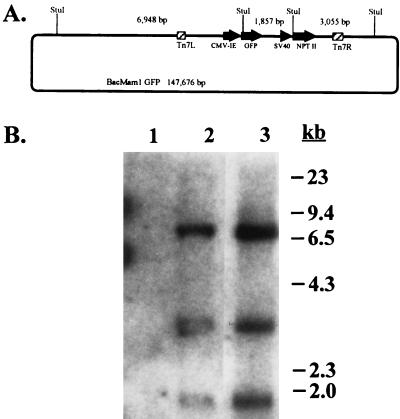
To analyze the DNA derived from recombinant virus maintained in CHO stable derivatives, cellular DNA was isolated from the pooled G418-resistant CHO cells described above and subjected to Southern blot analysis. The complete sequence of viral DNA present in BacMam1 GFP was assembled according to details in Luckow et al. (18). A cartoon showing the four StuI recognition sites around the recombinant expression cassette is shown in Fig. 7A; there are nine additional StuI sites in the genome. A Southern blot of StuI-digested DNA using pFastBacMam1 GFP DNA as probe is shown in Fig. 7B. The probe used in this experiment contains sequences between the Tn7 attachment site left and right ends as shown in Fig. 1; thus, three fragments of approximately 7 kbp, 3 kbp, and 2 kbp are expected from StuI digestion of recombinant BacMam1 GFP viral DNA (Fig. 7A) as is observed (Fig. 7B, lane 3). The identical result is obtained with DNA isolated from the pool of transduced CHO cells (Fig. 7B, lane 2) and from three clonal lines derived from this pool (data not shown). This indicates that the stable cell lines isolated from transduction with BacMam1 GFP virus harbor at least 12 kbp of DNA derived from the viral vector.
Figure 7.
Analysis of baculovirus-derived DNA in stably transduced CHO cells. (A) Map of BacMam1 GFP recombinant baculovirus DNA. StuI recognition sites internal and adjacent to the recombinant expression cassettes are shown with sizes of the expected fragments. Drawing is not to scale. (B) Southern blot of StuI-digested DNA. Details are described in the text. Lanes: 1, nontransduced cells; 2, transduced cells, selected pool; 3, BacMam1 GFP viral DNA.
In addition to CHO cells, a number of cell lines listed in Table 1 that had been transduced by BacMam1 GFP were cultured in selective media with G418. Stably transduced HuH-7, CHO, CV-1, Cos-7, HeLa, 293, K562, and P388D1 cells that express GFP also have been obtained. In addition, we have used baculovirus to stably transduce two other human hepatoma lines, HepG2 and Hep3B, as well as the BeWo chondrocytoma cell line.
Frequency of Stable Cell Line Formation.
As shown in Fig. 6A, G418-resistant colonies of CHO cells arise from cultures transduced at a moi of 1 pfu per cell. To measure the frequency, duplicate cultures of CHO cells were transduced at a low moi with BacMam1 GFP. One set of cultures was analyzed by using flow cytometry to determine the number of detectable green cells. The other set of cultures was selected with G418 until nontransduced control cells died and colonies were visible in transduced cultures. Colonies were subsequently stained with crystal violet and counted. Frequency of colony formation was obtained by dividing the number of transduced cells by the number of colonies obtained (Table 2). This yields frequencies that range from 1 clone in 39 transduced cells to 1 clone in 109 transduced cells, indicating that the formation of stable transductants is an efficient event.
Table 2.
Frequency of colony formation
| Moi | Green cells | G418R colonies | Frequency |
|---|---|---|---|
| 0 | 0 | 0 | — |
| 0.5 | 1,525 | 14 | 1/109 |
| 1 | 2,925 | 66 | 1/44 |
| 2 | 6,450 | 166 | 1/39 |
| 5 | 14,950 | 183 | 1/82 |
| 10 | 26,600 | TMTC | — |
Moi, multiplicity of infection. TMTC, too many to count.
DISCUSSION
Several reports have demonstrated the utility of recombinant baculoviruses as gene-delivery vehicles for transient expression of foreign genes in a limited number of mammalian cells (3–6). This report significantly expands the number of mammalian cell types that can be transduced to include several primary cell types as well as various continuous cell lines. The broad range of susceptible cells, coupled with the relative ease of construction and propagation of the virus, makes the baculovirus system a useful tool for studying expression and function of gene products by facilitating transfer of foreign genes. Additionally, this system can facilitate assessment of promoter strength and regulation across cells obtained from many different tissues.
Our results have further extended the value of this system in two significant aspects. First, addition of either butyrate or TSA to transduced cultures dramatically increases levels of transient gene expression directed from the described recombinant expression cassettes. The dose-dependent increase in gene expression, exhibited over two orders of magnitude of TSA concentration, allows for a system whereby levels of a particular gene product can be regulated by varying the concentration of TSA. Second, we have successfully used the baculovirus vector to derive cell lines that stably maintain and express a recombinant expression cassette. As demonstrated with human keratinocytes and bone marrow fibroblasts, the ability to effectively transduce primary cell types may prove extremely useful in attempting to derive valuable human cell lines following the transduction of establishment genes such as SV40 large T antigen. This may prove especially useful for cell types that are either difficult to isolate in large numbers or are difficult to transfect by other methods.
In contrast to replication-deficient viral gene delivery systems for mammalian cells that require the use of helper functions to propagate virus, the baculovirus system provides a good compromise. The virus is propagated in insect cells, yet is inherently unable to replicate in mammalian cells (refs. 1 and 2; J.P.C. and T.A.K., unpublished results), thus avoiding any risk of breakthrough of replication-competent virus. Currently, we are unsure as to the exact state of the baculovirus DNA that is maintained in the stable cell lines derived in this study. The data presented in Fig. 7 indicate that at least 12 kbp of viral DNA is maintained in pooled G418-resistant, GFP-expressing CHO cells. Further investigation will be needed to determine exactly how much of the viral genome can be maintained and whether it is integrated into the host genome. It is possible that the viral DNA exists as an extrachromosomal element in these cells, although this seems unlikely, because the DNA persists for at least 25 passages. Tjia et al. (19), using Southern blotting, could detect baculovirus DNA in nuclei of mammalian cells until 24–48 hr after treatment of cultures with virus at a moi of 100 but not at later times. They did not rule out the possibility that regions of the baculovirus DNA would become integrated into the genome of individual cells in the culture and would be undetectable unless such cells could be selected in some way. The persistence of large regions of the viral genome in cells, which we have demonstrated in this report, raises the possibility that immediate early viral gene products could be expressed in mammalian cells that receive a virus. One of these gene products, the IE-1 transactivator, has been demonstrated to activate transcription from viral early promoters in mammalian cells (20).
Acknowledgments
The authors thank Ruth Lightfoot, Jacqueline Lee, Janet Parham, Walt Dallas, Kathy Kilpatrick, and Greg Pahel for their contributions to this manuscript. We also acknowledge these investigators for providing cell lines: Julie Barnes, Avis Bridgers, Lynn Condreay, Lorrie Delehanty, Kirby Gottschalk, Jill Haizlip, Channa Jayawickreme, Bob Johnston (University of North Carolina, Chapel Hill), Bob Mertz, Staton Noel, Janet Parham, Jeff Pfohl, Pam Watkins, Michael Watson, and the Glaxo Wellcome BioResources staff.
ABBREVIATIONS
- CMV-IE
cytomegalovirus immediate early
- NPT II
neomycin phosphotransferase II
- GFP
green fluorescent protein
- TSA
trichostatin A
- moi
multiplicity of infection
- pfu
plaque forming units
- SV40
simian virus 40
- CHO
Chinese hamster ovary
References
- 1.Volkman L E, Goldsmith P A. Appl Environ Microbiol. 1983;45:1085–1093. doi: 10.1128/aem.45.3.1085-1093.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbonell L F, Miller L K. Appl Environ Microbiol. 1987;53:1412–1417. doi: 10.1128/aem.53.7.1412-1417.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Proc Natl Acad Sci USA. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyce F M, Bucher N L. Proc Natl Acad Sci USA. 1996;93:2348–2352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoji I, Aizaki H, Tani H, Ishii K, Chiba T, Saito I, Miyamura T, Matsuura Y. J Gen Virol. 1997;78:2657–2664. doi: 10.1099/0022-1317-78-10-2657. [DOI] [PubMed] [Google Scholar]
- 6.Yap C-C, Ishii K, Aoki Y, Aizaki H, Tani H, Shimizu H, Ueno Y, Miyamura T, Matsuura Y. Virology. 1997;231:192–200. doi: 10.1006/viro.1997.8537. [DOI] [PubMed] [Google Scholar]
- 7.O’Reilly D R, Miller L K, Luckow V A. Baculovirus Expression Vectors: A Laboratory Manual. New York: Oxford Univ. Press; 1992. [Google Scholar]
- 8.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 9.Jeon S, Allen-Hoffmann B L, Lambert P F. J Virol. 1995;69:2989–2997. doi: 10.1128/jvi.69.5.2989-2997.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 11.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 12.Kruh J. Mol Cell Biochem. 1982;42:65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- 13.Volkman L E, Goldsmith P A. Virology. 1985;143:185–195. doi: 10.1016/0042-6822(85)90107-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Hammer D A, Granados R R. J Gen Virol. 1997;78:3081–3089. doi: 10.1099/0022-1317-78-12-3081. [DOI] [PubMed] [Google Scholar]
- 15.Gluzman Y. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida M, Horinouchi S, Beppu T. BioEssays. 1995;17:423–430. doi: 10.1002/bies.950170510. [DOI] [PubMed] [Google Scholar]
- 17.Ganem D. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott; 1996. pp. 2703–2737. [Google Scholar]
- 18.Luckow V A, Lee S C, Barry G F, Olins P O. J Virol. 1993;67:4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tjia S T, zu Altenschildesche G M, Doerfler W. Virology. 1983;125:107–117. doi: 10.1016/0042-6822(83)90067-3. [DOI] [PubMed] [Google Scholar]
- 20.Murges D, Kremer A, Knebel-Morsdorf D. J Gen Virol. 1997;78:1507–1510. doi: 10.1099/0022-1317-78-6-1507. [DOI] [PubMed] [Google Scholar]