Abstract
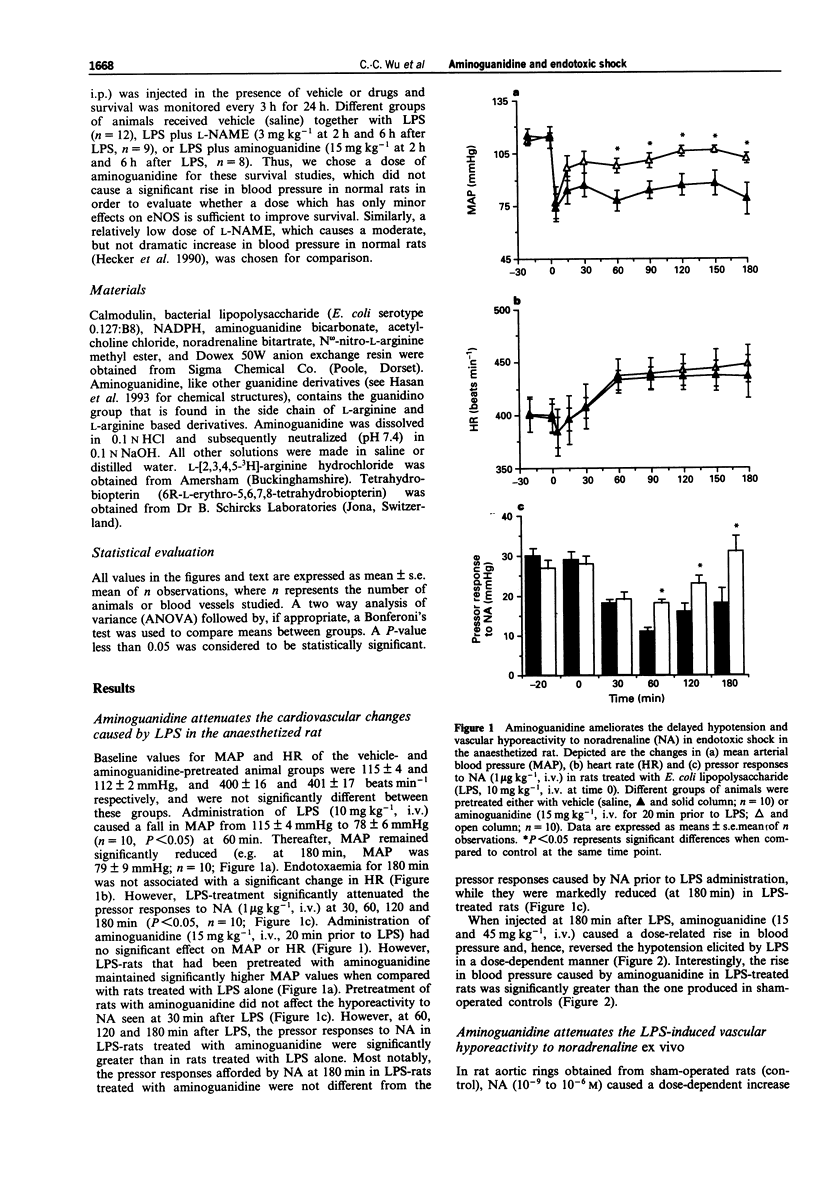
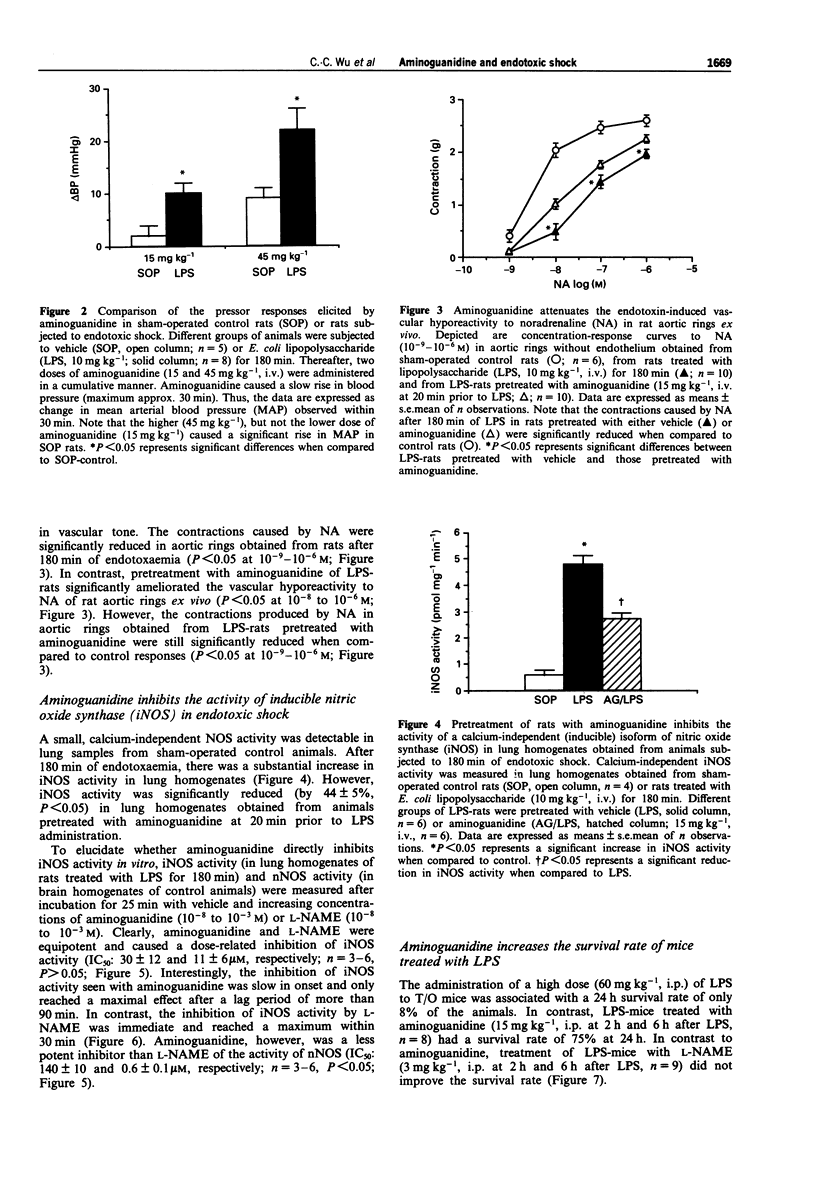
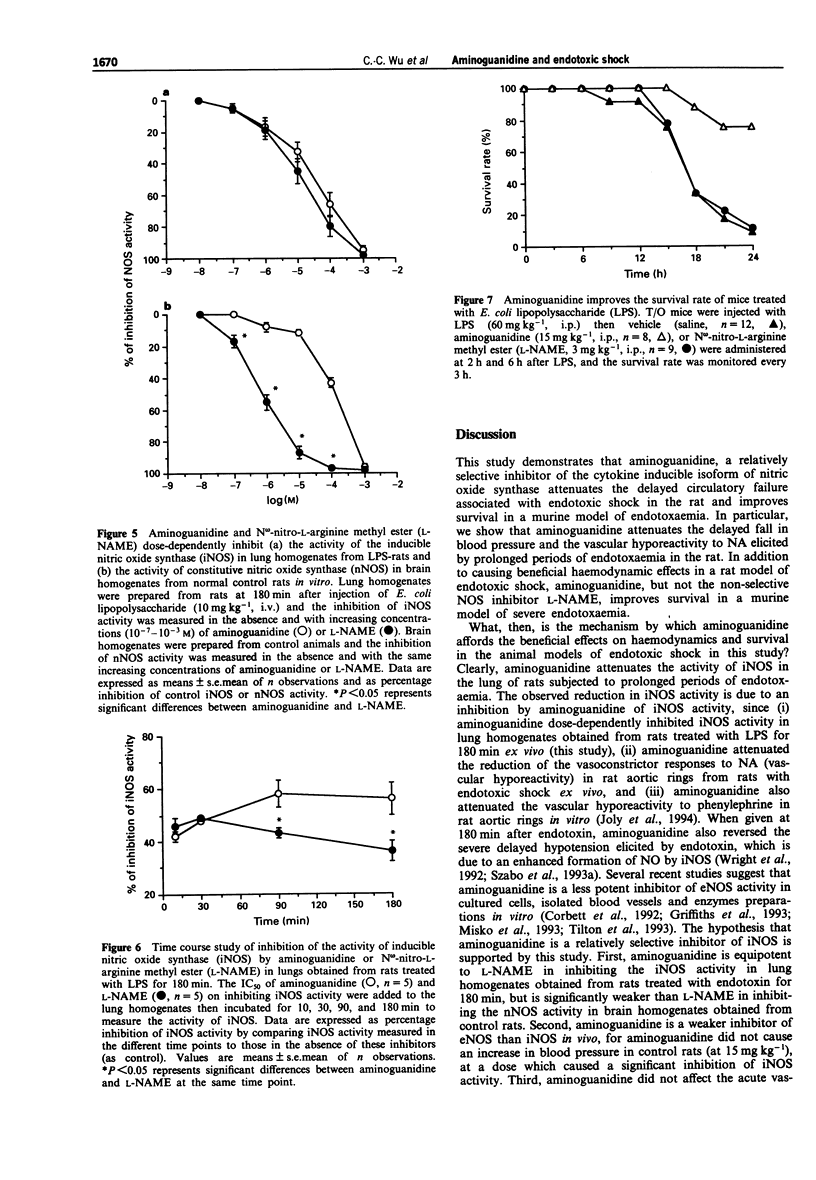
1. We have investigated the effects of aminoguanidine, a relatively selective inhibitor of the cytokine-inducible isoform of nitric oxide synthase (iNOS), on the delayed circulatory failure, vascular hyporeactivity to vasoconstrictor agents, and iNOS activity in a rat model of circulatory shock induced by bacterial endotoxin (E. coli lipopolysaccharide; LPS). In addition, we have evaluated the effect of aminoguanidine on the 24 h survival rate in a murine model of endotoxaemia. 2. Male Wistar rats were anaesthetized and instrumented for the measurement of mean arterial blood pressure (MAP) and heart rate (HR). Injection of LPS (10 mg kg-1, i.v.) resulted in a fall in MAP from 115 +/- 4 mmHg (time 0, control) to 79 +/- 9 mmHg at 180 min (P < 0.05, n = 10). The pressor effect of noradrenaline (NA, 1 microgram kg-1, i.v.) was also significantly reduced at 60, 120 and 180 min after LPS injection. In contrast, animals pretreated with aminoguanidine (15 mg kg-1, i.v., 20 min prior to LPS injection) maintained a significantly higher MAP (at 180 min, 102 +/- 3 mmHg, n = 10, P < 0.05) when compared to rats given only LPS (LPS-rats). Cumulative administration of aminoguanidine (15 mg kg-1 and 45 mg kg-1) given 180 min after LPS caused a dose-related increase in MAP and reversed the hypotension. Aminoguanidine also significantly alleviated the reduction of the pressor response to NA: indeed, at 180 min, the pressor response returned to normal in aminoguanidine pretreated LPS-rats.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biegański T., Kusche J., Lorenz W., Hesterberg R., Stahlknecht C. D., Feussner K. D. Distribution and properties of human intestinal diamine oxidase and its relevance for the histamine catabolism. Biochim Biophys Acta. 1983 Mar 31;756(2):196–203. doi: 10.1016/0304-4165(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Dinerman J. L., Lowenstein C. J., Snyder S. H. Molecular mechanisms of nitric oxide regulation. Potential relevance to cardiovascular disease. Circ Res. 1993 Aug;73(2):217–222. doi: 10.1161/01.res.73.2.217. [DOI] [PubMed] [Google Scholar]
- Edelstein D., Brownlee M. Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine. Diabetes. 1992 Jan;41(1):26–29. doi: 10.2337/diab.41.1.26. [DOI] [PubMed] [Google Scholar]
- Griffiths M. J., Messent M., MacAllister R. J., Evans T. W. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br J Pharmacol. 1993 Nov;110(3):963–968. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbrecht B. G., Billiar T. R., Stadler J., Demetris A. J., Ochoa J., Curran R. D., Simmons R. L. Inhibition of nitric oxide synthesis during endotoxemia promotes intrahepatic thrombosis and an oxygen radical-mediated hepatic injury. J Leukoc Biol. 1992 Oct;52(4):390–394. doi: 10.1002/jlb.52.4.390. [DOI] [PubMed] [Google Scholar]
- Hasan K., Heesen B. J., Corbett J. A., McDaniel M. L., Chang K., Allison W., Wolffenbuttel B. H., Williamson J. R., Tilton R. G. Inhibition of nitric oxide formation by guanidines. Eur J Pharmacol. 1993 Nov 2;249(1):101–106. doi: 10.1016/0014-2999(93)90667-7. [DOI] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Harris H. J., Katsura M., Thiemermann C., Vane J. R. Endothelial cells metabolize NG-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1037–1043. doi: 10.1016/0006-291x(90)90627-y. [DOI] [PubMed] [Google Scholar]
- Hutcheson I. R., Whittle B. J., Boughton-Smith N. K. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol. 1990 Dec;101(4):815–820. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly G. A., Ayres M., Chelly F., Kilbourn R. G. Effects of NG-methyl-L-arginine, NG-nitro-L-arginine, and aminoguanidine on constitutive and inducible nitric oxide synthase in rat aorta. Biochem Biophys Res Commun. 1994 Feb 28;199(1):147–154. doi: 10.1006/bbrc.1994.1207. [DOI] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Griffith O. W. Overproduction of nitric oxide in cytokine-mediated and septic shock. J Natl Cancer Inst. 1992 Jun 3;84(11):827–831. doi: 10.1093/jnci/84.11.827. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Gross S. S., Jubran A., Adams J., Griffith O. W., Levi R., Lodato R. F. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci U S A. 1990 May;87(9):3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Traber L. D., Nelson S., Lentz C. W., Nakazawa H., Herndon D. N., Noda H., Traber D. L. Reversal of hyperdynamic response to continuous endotoxin administration by inhibition of NO synthesis. J Appl Physiol (1985) 1992 Jul;73(1):324–328. doi: 10.1152/jappl.1992.73.1.324. [DOI] [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Ou P., Wolff S. P. Aminoguanidine: a drug proposed for prophylaxis in diabetes inhibits catalase and generates hydrogen peroxide in vitro. Biochem Pharmacol. 1993 Oct 5;46(7):1139–1144. doi: 10.1016/0006-2952(93)90461-5. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B. The influence of catabolic reactions on polyamine excretion. Biochem J. 1985 Jan 1;225(1):219–226. doi: 10.1042/bj2250219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz P. J., Raij L. Endogenously synthesized nitric oxide prevents endotoxin-induced glomerular thrombosis. J Clin Invest. 1992 Nov;90(5):1718–1725. doi: 10.1172/JCI116045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suba E. A., McKenna T. M., Williams T. J. In vivo and in vitro effects of endotoxin on vascular responsiveness to norepinephrine and signal transduction in the rat. Circ Shock. 1992 Feb;36(2):127–133. [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Thiemermann C., Vane J. R. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C., Wu C. C., Gross S. S., Thiemermann C., Vane J. R. Interleukin-1 contributes to the induction of nitric oxide synthase by endotoxin in vivo. Eur J Pharmacol. 1993 Nov 30;250(1):157–160. doi: 10.1016/0014-2999(93)90634-t. [DOI] [PubMed] [Google Scholar]
- Szabó C., Wu C. C., Mitchell J. A., Gross S. S., Thiemermann C., Vane J. R. Platelet-activating factor contributes to the induction of nitric oxide synthase by bacterial lipopolysaccharide. Circ Res. 1993 Dec;73(6):991–999. doi: 10.1161/01.res.73.6.991. [DOI] [PubMed] [Google Scholar]
- Thiemermann C. The role of the L-arginine: nitric oxide pathway in circulatory shock. Adv Pharmacol. 1994;28:45–79. doi: 10.1016/s1054-3589(08)60493-7. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Wu C. C., Szabó C., Perretti M., Vane J. R. Role of tumour necrosis factor in the induction of nitric oxide synthase in a rat model of endotoxin shock. Br J Pharmacol. 1993 Sep;110(1):177–182. doi: 10.1111/j.1476-5381.1993.tb13789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton R. G., Chang K., Hasan K. S., Smith S. R., Petrash J. M., Misko T. P., Moore W. M., Currie M. G., Corbett J. A., McDaniel M. L. Prevention of diabetic vascular dysfunction by guanidines. Inhibition of nitric oxide synthase versus advanced glycation end-product formation. Diabetes. 1993 Feb;42(2):221–232. doi: 10.2337/diab.42.2.221. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- Wu C. C., Szabó C., Chen S. J., Thiemermann C., Vane J. R. Activation of soluble guanylyl cyclase by a factor other than nitric oxide or carbon monoxide contributes to the vascular hyporeactivity to vasoconstrictor agents in the aorta of rats treated with endotoxin. Biochem Biophys Res Commun. 1994 May 30;201(1):436–442. doi: 10.1006/bbrc.1994.1720. [DOI] [PubMed] [Google Scholar]


