Abstract
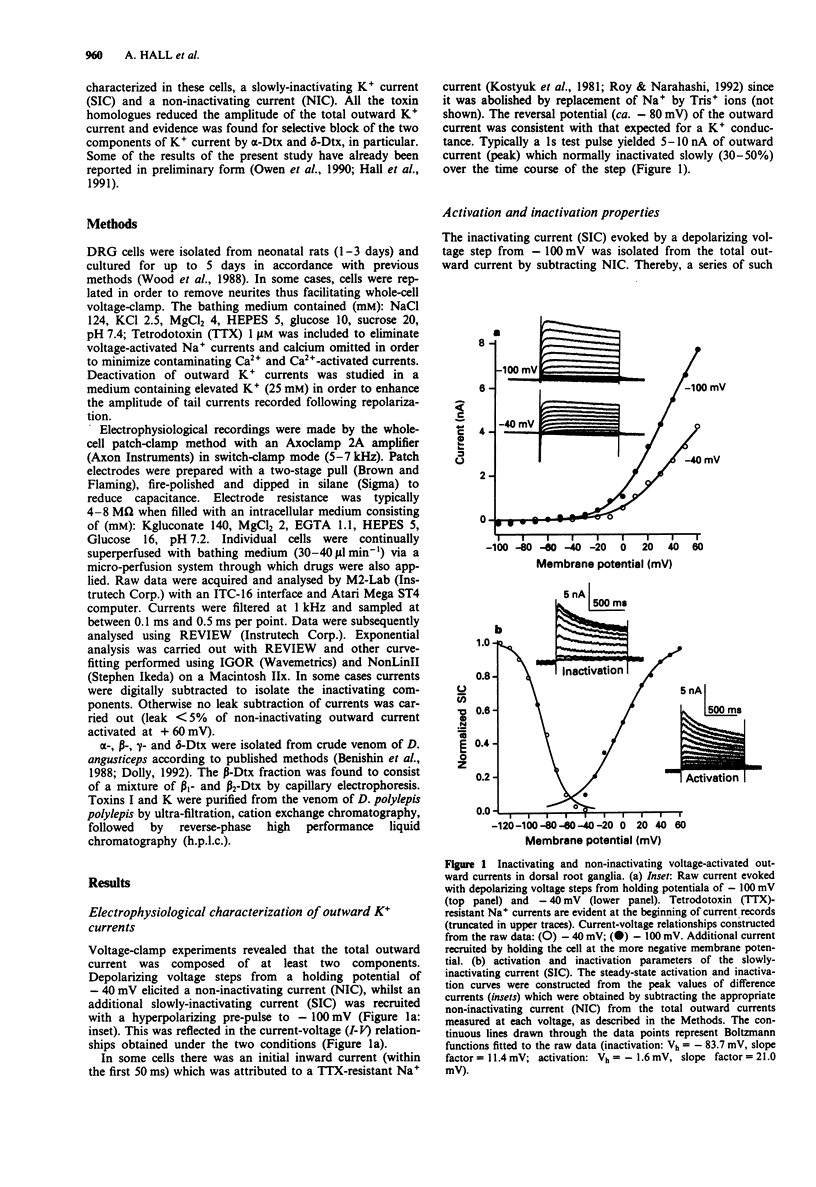
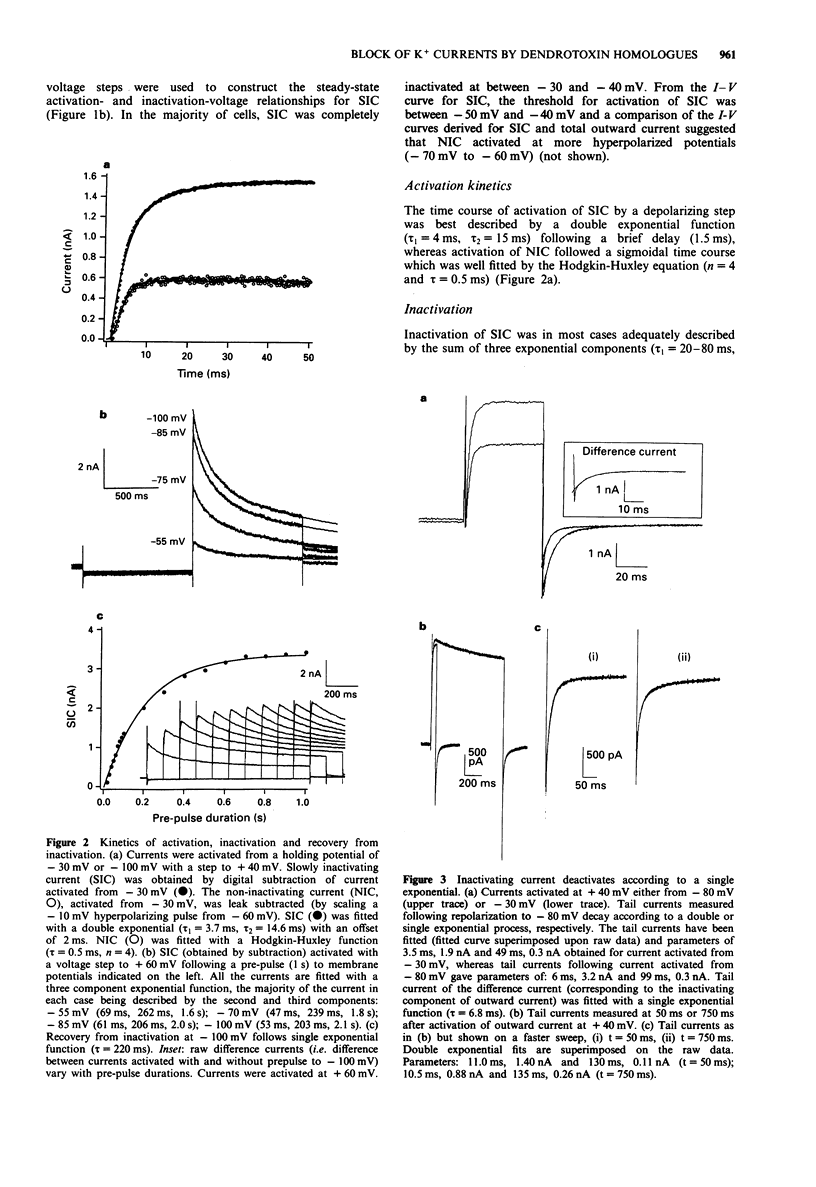
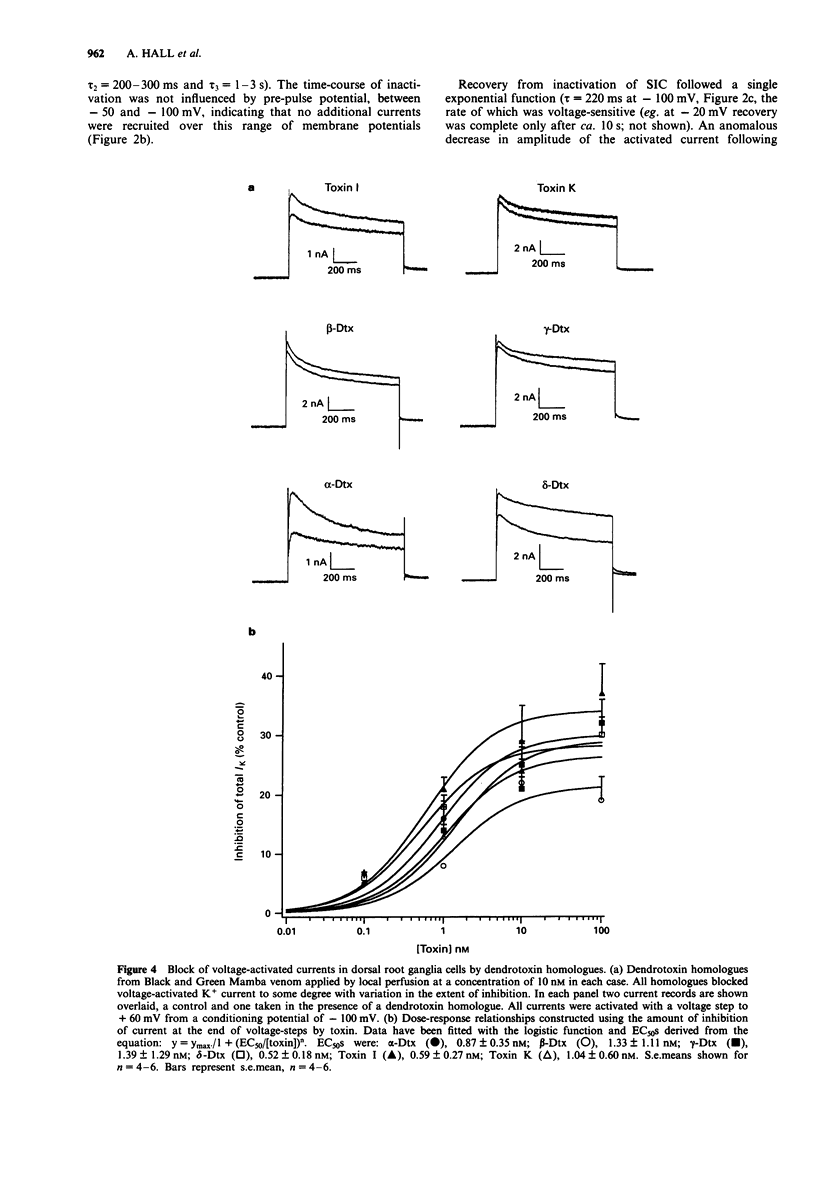
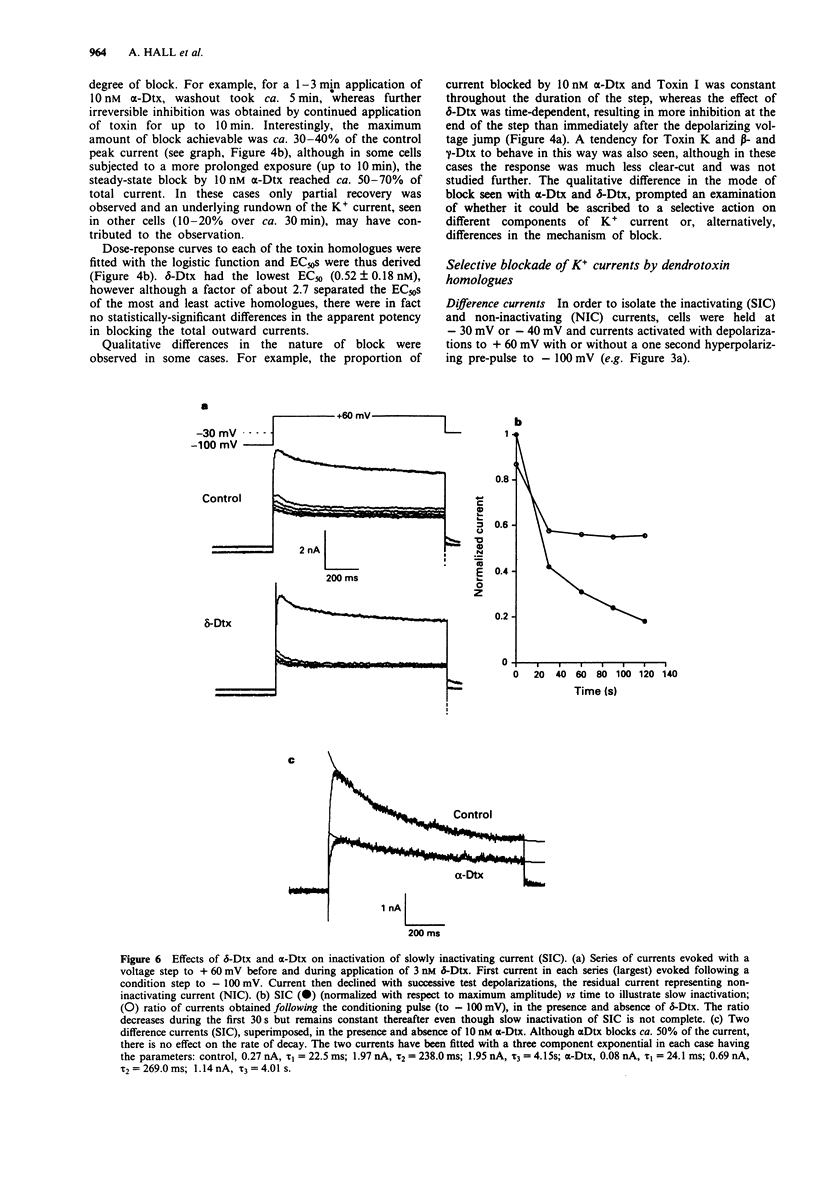
1. Homologues of dendrotoxin (Dtx) were isolated from the crude venom of Green and Black Mamba snakes and examined for K+ channel blocking activity in neonatal rat dorsal root ganglion cells (DRGs) by whole-cell patch clamp recording. 2. Outward potassium current activated by depolarization was composed of two major components: a slowly inactivating current (SIC, tau decay approximately 50 ms, 200 ms and 2s), and a non-inactivating current (NIC, tau decay > 2 min). Tail current analysis revealed two time constants of deactivation of total outward current, 3-12 ms and 50-150 ms (at -80 mV) which corresponded to SIC and NIC, respectively. 3. All the homologues (alpha-, beta-, gamma- and delta-Dtx and toxins I and K) blocked outward current activated by depolarization in a dose-dependent manner. The most potent in blocking total outward current was delta-Dtx (EC50 of 0.5 +/- 0.2 nM), although there were no statistically significant differences in potency between any of the homologues. 4. Qualitative differences in the nature of the block were noted between homologues. In particular, the block by delta-Dtx was time-dependent, whereas that by alpha-Dtx was not. 5. alpha-Dtx was a much better blocker of SIC (EC50 = 1.0 +/- 0.4 nM) than was delta-Dtx (EC50 = 17.6 +/- 5.8 nM). Furthermore, delta-Dtx was selective for NIC (EC50 +/- 0.24 +/- 0.03 nM) over SIC and reduced the slow component of tail currents (NIC), preferentially.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awan K. A., Dolly J. O. K+ channel sub-types in rat brain: characteristic locations revealed using beta-bungarotoxin, alpha- and delta-dendrotoxins. Neuroscience. 1991;40(1):29–39. doi: 10.1016/0306-4522(91)90172-k. [DOI] [PubMed] [Google Scholar]
- Beckh S., Pongs O. Members of the RCK potassium channel family are differentially expressed in the rat nervous system. EMBO J. 1990 Mar;9(3):777–782. doi: 10.1002/j.1460-2075.1990.tb08173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishin C. G., Sorensen R. G., Brown W. E., Krueger B. K., Blaustein M. P. Four polypeptide components of green mamba venom selectively block certain potassium channels in rat brain synaptosomes. Mol Pharmacol. 1988 Aug;34(2):152–159. [PubMed] [Google Scholar]
- Benoit E., Dubois J. M. Toxin I from the snake Dendroaspis polylepis polylepis: a highly specific blocker of one type of potassium channel in myelinated nerve fiber. Brain Res. 1986 Jul 9;377(2):374–377. doi: 10.1016/0006-8993(86)90884-x. [DOI] [PubMed] [Google Scholar]
- Black A. R., Donegan C. M., Denny B. J., Dolly J. O. Solubilization and physical characterization of acceptors for dendrotoxin and beta-bungarotoxin from synaptic membranes of rat brain. Biochemistry. 1988 Sep 6;27(18):6814–6820. doi: 10.1021/bi00418a025. [DOI] [PubMed] [Google Scholar]
- Bosma M. M., Allen M. L., Martin T. M., Tempel B. L. PKA-dependent regulation of mKv1.1, a mouse Shaker-like potassium channel gene, when stably expressed in CHO cells. J Neurosci. 1993 Dec;13(12):5242–5250. doi: 10.1523/JNEUROSCI.13-12-05242.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräu M. E., Dreyer F., Jonas P., Repp H., Vogel W. A K+ channel in Xenopus nerve fibres selectively blocked by bee and snake toxins: binding and voltage-clamp experiments. J Physiol. 1990 Jan;420:365–385. doi: 10.1113/jphysiol.1990.sp017918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufton M. J. Proteinase inhibitors and dendrotoxins. Sequence classification, structural prediction and structure/activity. Eur J Biochem. 1985 Dec 16;153(3):647–654. doi: 10.1111/j.1432-1033.1985.tb09349.x. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Othman I. B., Pelchen-Matthews A., Dolly J. O. Central action of dendrotoxin: selective reduction of a transient K conductance in hippocampus and binding to localized acceptors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):493–497. doi: 10.1073/pnas.83.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. L., Karlsson E. Dendrotoxin from the venom of the green mamba, Dendroaspis angusticeps. A neurotoxin that enhances acetylcholine release at neuromuscular junction. Naunyn Schmiedebergs Arch Pharmacol. 1980 May;312(1):1–6. doi: 10.1007/BF00502565. [DOI] [PubMed] [Google Scholar]
- Harvey A. L., Karlsson E. Protease inhibitor homologues from mamba venoms: facilitation of acetylcholine release and interactions with prejunctional blocking toxins. Br J Pharmacol. 1982 Sep;77(1):153–161. doi: 10.1111/j.1476-5381.1982.tb09281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollecker M., Marshall D. L., Harvey A. L. Structural features important for the biological activity of the potassium channel blocking dendrotoxins. Br J Pharmacol. 1993 Oct;110(2):790–794. doi: 10.1111/j.1476-5381.1993.tb13881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. S., Benishin C., Fredholm B. B. Comparison of the effects of four dendrotoxin peptides, 4-aminopyridine and tetraethylammonium on the electrically evoked [3H]noradrenaline release from rat hippocampus. Eur J Pharmacol. 1991 Dec 10;209(1-2):87–93. doi: 10.1016/0014-2999(91)90015-i. [DOI] [PubMed] [Google Scholar]
- Joubert F. J., Taljaard N. Snake venoms. The amino acid sequences of two proteinase inhibitor homologues from Dendroaspis angusticeps venom. Hoppe Seylers Z Physiol Chem. 1980 May;361(5):661–674. doi: 10.1515/bchm2.1980.361.1.661. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-I. Sodium currents. Neuroscience. 1981;6(12):2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Muniz Z. M., Diniz C. R., Dolly J. O. Characterisation of binding sites for delta-dendrotoxin in guinea-pig synaptosomes: relationship to acceptors for the K+-channel probe alpha-dendrotoxin. J Neurochem. 1990 Jan;54(1):343–346. doi: 10.1111/j.1471-4159.1990.tb13320.x. [DOI] [PubMed] [Google Scholar]
- Parcej D. N., Dolly J. O. Dendrotoxin acceptor from bovine synaptic plasma membranes. Binding properties, purification and subunit composition of a putative constituent of certain voltage-activated K+ channels. Biochem J. 1989 Feb 1;257(3):899–903. doi: 10.1042/bj2570899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A., Dolly J. O. Distribution in the rat central nervous system of acceptor sub-types for dendrotoxin, a K+ channel probe. Neuroscience. 1989;29(2):347–361. doi: 10.1016/0306-4522(89)90062-6. [DOI] [PubMed] [Google Scholar]
- Penner R., Petersen M., Pierau F. K., Dreyer F. Dendrotoxin: a selective blocker of a non-inactivating potassium current in guinea-pig dorsal root ganglion neurones. Pflugers Arch. 1986 Oct;407(4):365–369. doi: 10.1007/BF00652619. [DOI] [PubMed] [Google Scholar]
- Po S., Roberds S., Snyders D. J., Tamkun M. M., Bennett P. B. Heteromultimeric assembly of human potassium channels. Molecular basis of a transient outward current? Circ Res. 1993 Jun;72(6):1326–1336. doi: 10.1161/01.res.72.6.1326. [DOI] [PubMed] [Google Scholar]
- Rehm H., Lazdunski M. Purification and subunit structure of a putative K+-channel protein identified by its binding properties for dendrotoxin I. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4919–4923. doi: 10.1073/pnas.85.13.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm H., Newitt R. A., Tempel B. L. Immunological evidence for a relationship between the dendrotoxin-binding protein and the mammalian homologue of the Drosophila Shaker K+ channel. FEBS Lett. 1989 Jun 5;249(2):224–228. doi: 10.1016/0014-5793(89)80628-3. [DOI] [PubMed] [Google Scholar]
- Reid P. F., Pongs O., Dolly J. O. Cloning of a bovine voltage-gated K+ channel gene utilising partial amino acid sequence of a dendrotoxin-binding protein from brain cortex. FEBS Lett. 1992 May 4;302(1):31–34. doi: 10.1016/0014-5793(92)80277-n. [DOI] [PubMed] [Google Scholar]
- Robertson B., Owen D. G. Pharmacology of a cloned potassium channel from mouse brain (MK-1) expressed in CHO cells: effects of blockers and an 'inactivation peptide'. Br J Pharmacol. 1993 Jul;109(3):725–735. doi: 10.1111/j.1476-5381.1993.tb13634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M. L., Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci. 1992 Jun;12(6):2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott V. E., Muniz Z. M., Sewing S., Lichtinghagen R., Parcej D. N., Pongs O., Dolly J. O. Antibodies specific for distinct Kv subunits unveil a heterooligomeric basis for subtypes of alpha-dendrotoxin-sensitive K+ channels in bovine brain. Biochemistry. 1994 Feb 22;33(7):1617–1623. doi: 10.1021/bi00173a001. [DOI] [PubMed] [Google Scholar]
- Scott V. E., Parcej D. N., Keen J. N., Findlay J. B., Dolly J. O. Alpha-dendrotoxin acceptor from bovine brain is a K+ channel protein. Evidence from the N-terminal sequence of its larger subunit. J Biol Chem. 1990 Nov 25;265(33):20094–20097. [PubMed] [Google Scholar]
- Sheng M., Liao Y. J., Jan Y. N., Jan L. Y. Presynaptic A-current based on heteromultimeric K+ channels detected in vivo. Nature. 1993 Sep 2;365(6441):72–75. doi: 10.1038/365072a0. [DOI] [PubMed] [Google Scholar]
- Skarzyński T. Crystal structure of alpha-dendrotoxin from the green mamba venom and its comparison with the structure of bovine pancreatic trypsin inhibitor. J Mol Biol. 1992 Apr 5;224(3):671–683. doi: 10.1016/0022-2836(92)90552-u. [DOI] [PubMed] [Google Scholar]
- Sorensen R. G., Schneider M. J., Rogowski R. S., Blaustein M. P. Snake and scorpion neurotoxins as probes of rat brain synaptosomal potassium channels. Prog Clin Biol Res. 1990;334:279–301. [PubMed] [Google Scholar]
- Stansfeld C. E., Marsh S. J., Halliwell J. V., Brown D. A. 4-Aminopyridine and dendrotoxin induce repetitive firing in rat visceral sensory neurones by blocking a slowly inactivating outward current. Neurosci Lett. 1986 Mar 14;64(3):299–304. doi: 10.1016/0304-3940(86)90345-9. [DOI] [PubMed] [Google Scholar]
- Stansfeld C., Feltz A. Dendrotoxin-sensitive K+ channels in dorsal root ganglion cells. Neurosci Lett. 1988 Oct 31;93(1):49–55. doi: 10.1016/0304-3940(88)90011-0. [DOI] [PubMed] [Google Scholar]
- Strydom D. J. Snake venom toxins. Purification and properties of low-molecular-weight polypeptides of Dendroaspis polylepis polylepis (black mamba) venom. Eur J Biochem. 1976 Oct 1;69(1):169–176. doi: 10.1111/j.1432-1033.1976.tb10870.x. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kunkel D. D., Martin T. M., Schwartzkroin P. A., Tempel B. L. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993 Sep 2;365(6441):75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Winter J., James I. F., Rang H. P., Yeats J., Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988 Sep;8(9):3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


