Abstract
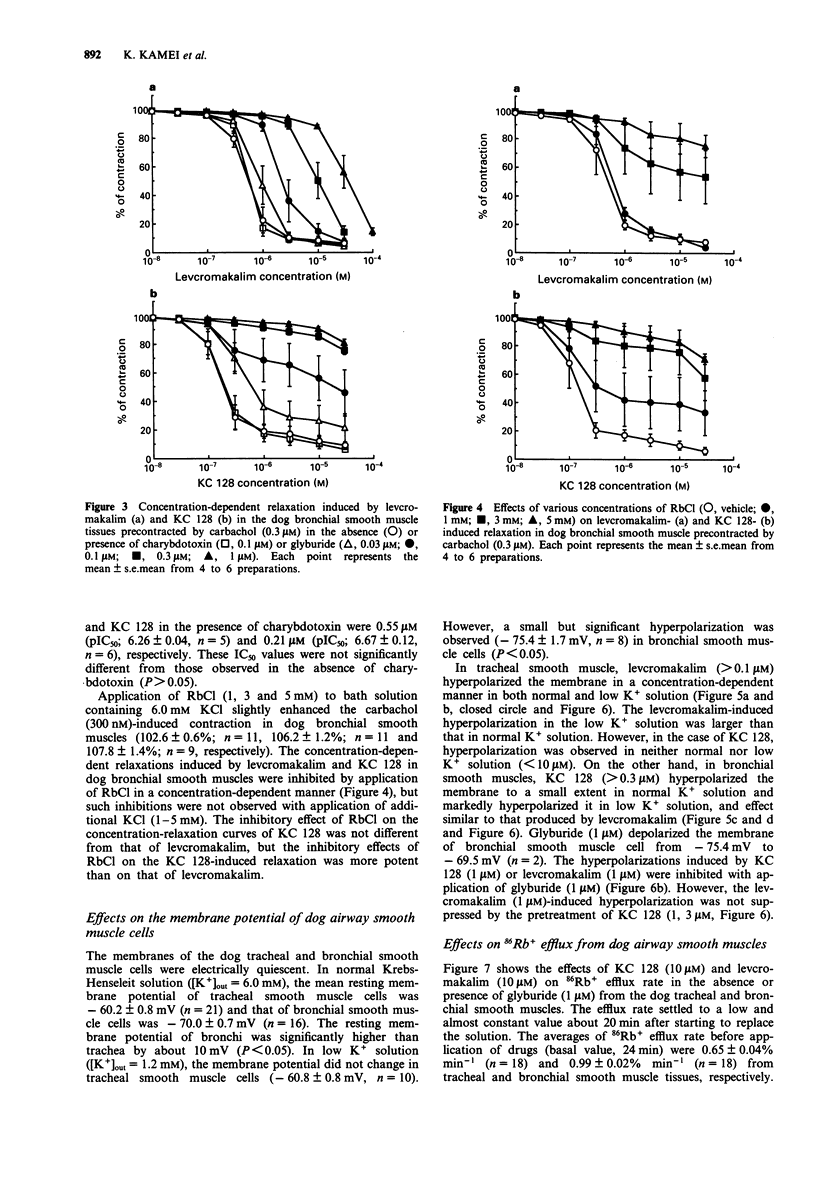
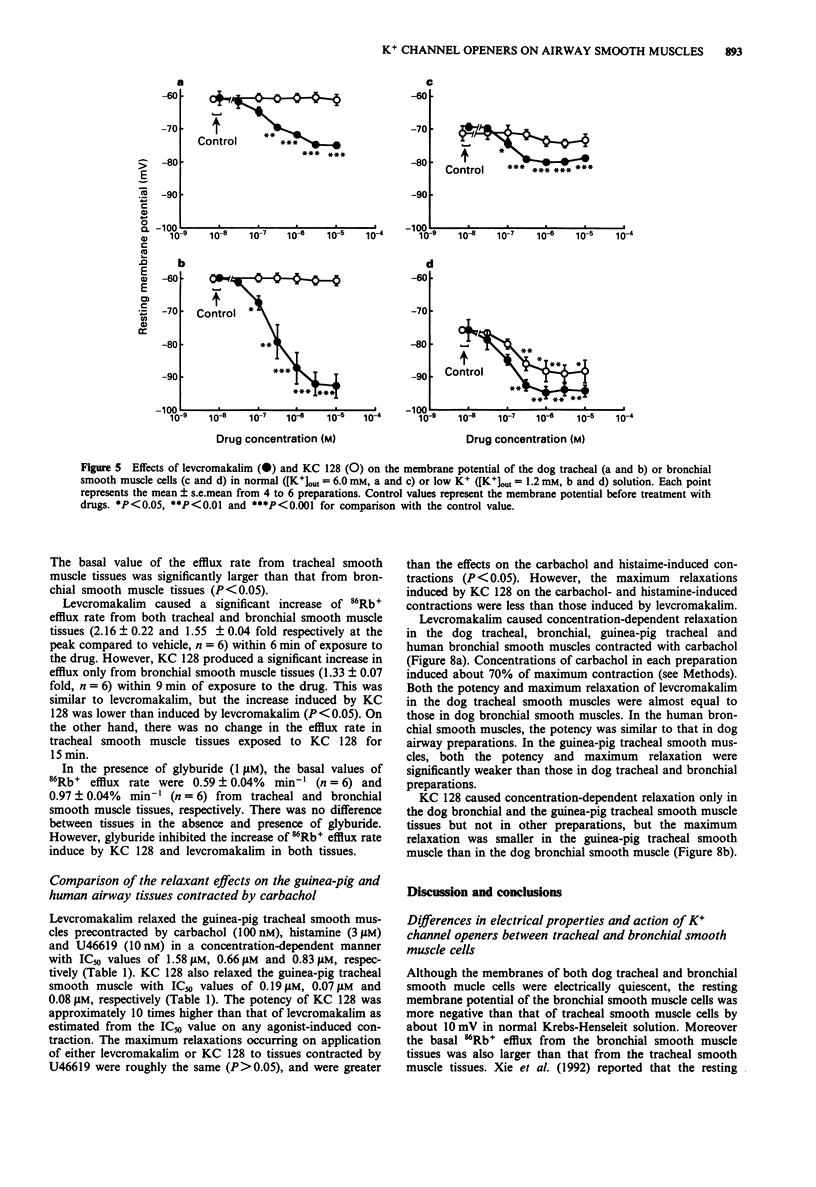
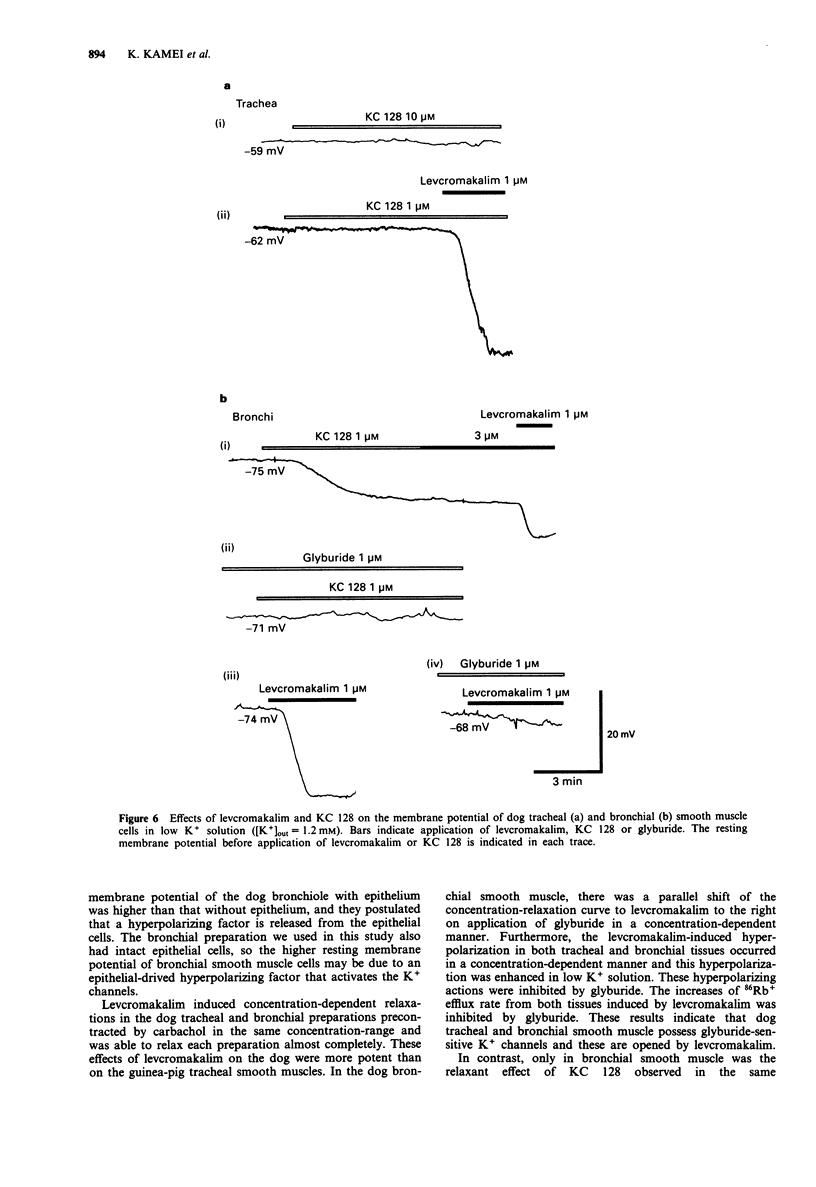
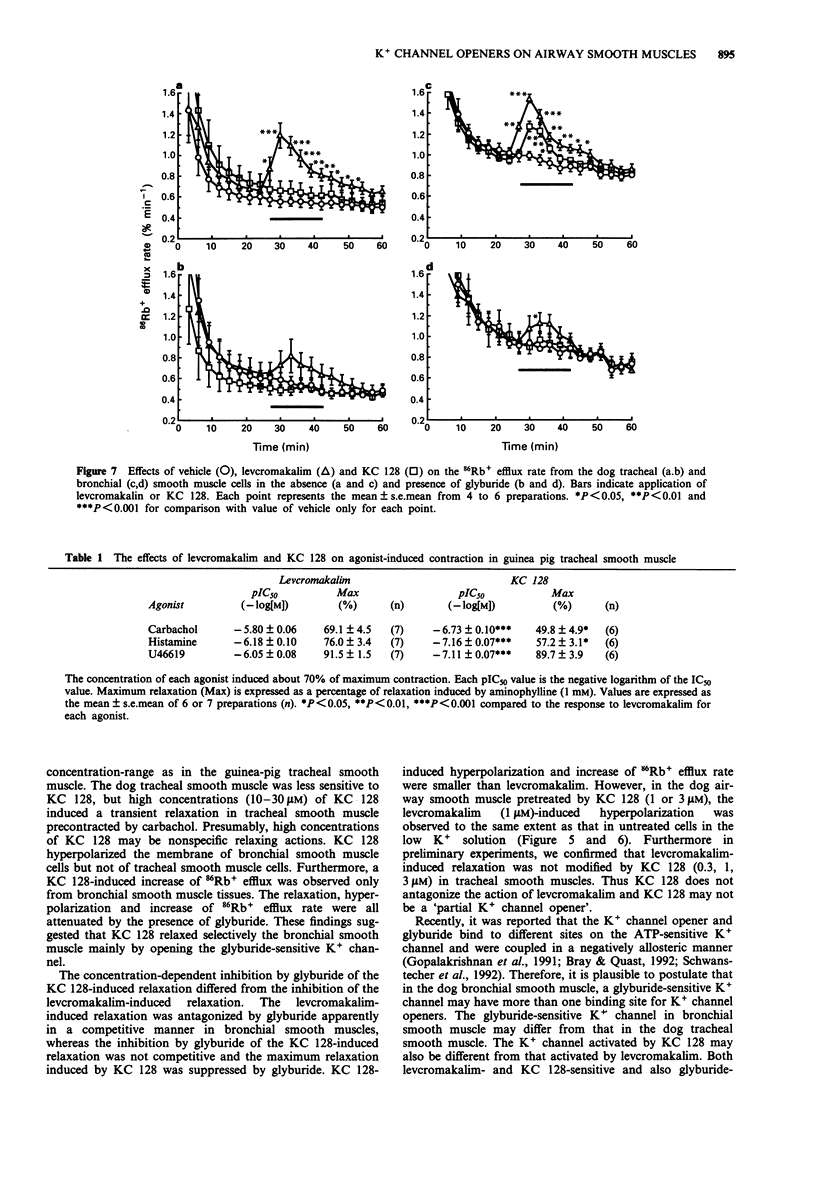
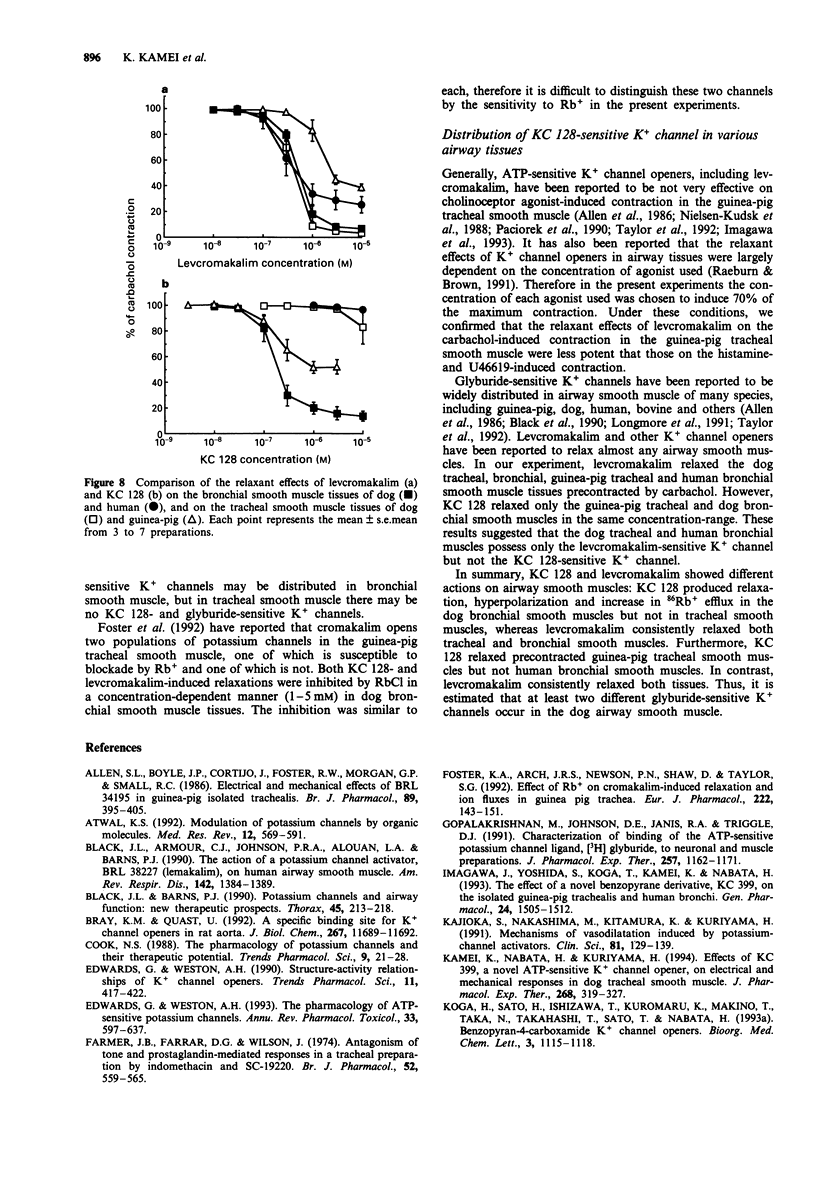
1. The purpose of the present experiments was to elucidate the differences in actions of two K+ channel openers, KC 128 and levcromakalim, on the carbachol-induced contraction, membrane potential and 86Rb+ efflux of the dog tracheal and bronchial smooth muscles. Furthermore, we compared the effects of these agents on guinea-pig and human airway smooth muscles. 2. In the dog tracheal and bronchial smooth muscle tissues, levcromakalim induced a concentration-dependent relaxation of the carbachol-induced contraction. The IC50 values were 0.35 microM (pIC50: 6.46 +/- 0.10, n = 9) and 0.55 microM (pIC50: 6.26 +/- 0.07, n = 5), respectively. KC 128 relaxed bronchial smooth muscles precontracted by carbachol with an IC50 value of 0.19 microM (pIC50: 6.73 +/- 0.10, n = 7). However, KC 128 had almost no effect on the contraction evoked by carbachol in the trachea (IC50 > 10 microM). The relaxations induced by levcromakalim and KC 128 were antagonized by glyburide (0.03-1 microM) but not by charybdotoxin (100 nM). 3. Levcromakalim (1 microM) hyperpolarized the membrane of both dog tracheal and bronchial smooth muscle cells, whereas KC 128 (1 microM) hyperpolarized the membrane of bronchial but not of tracheal smooth muscle cells. 4. Levcromakalim (10 microM) increased 86Rb+ efflux rate from both tracheal and bronchial smooth muscle tissues but KC 128 (10 microM) increased 86Rb+ efflux rate only from bronchial and not tracheal smooth muscle tissues. Glyburide (1 microM) prevented the hyperpolarization and the 86Rb+ efflux induced by these agents at the same concentration as observed for mechanical responses.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. L., Boyle J. P., Cortijo J., Foster R. W., Morgan G. P., Small R. C. Electrical and mechanical effects of BRL34915 in guinea-pig isolated trachealis. Br J Pharmacol. 1986 Oct;89(2):395–405. doi: 10.1111/j.1476-5381.1986.tb10273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal K. S. Modulation of potassium channels by organic molecules. Med Res Rev. 1992 Nov;12(6):569–591. doi: 10.1002/med.2610120603. [DOI] [PubMed] [Google Scholar]
- Black J. L., Armour C. L., Johnson P. R., Alouan L. A., Barnes P. J. The action of a potassium channel activator, BRL 38227 (lemakalim), on human airway smooth muscle. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1384–1389. doi: 10.1164/ajrccm/142.6_Pt_1.1384. [DOI] [PubMed] [Google Scholar]
- Black J. L., Barnes P. J. Potassium channels and airway function: new therapeutic prospects. Thorax. 1990 Mar;45(3):213–218. doi: 10.1136/thx.45.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray K. M., Quast U. A specific binding site for K+ channel openers in rat aorta. J Biol Chem. 1992 Jun 15;267(17):11689–11692. [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. Structure-activity relationships of K+ channel openers. Trends Pharmacol Sci. 1990 Oct;11(10):417–422. doi: 10.1016/0165-6147(90)90149-3. [DOI] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- Farmer J. B., Farrar D. G., Wilson J. Antagonism of tone and prostaglandin-mediated responses in a tracheal preparation by indomethacin and SC-19220. Br J Pharmacol. 1974 Dec;52(4):559–565. doi: 10.1111/j.1476-5381.1974.tb09724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K. A., Arch J. R., Newson P. N., Shaw D., Taylor S. G. Effect of Rb+ on cromakalim-induced relaxation and ion fluxes in guinea pig trachea. Eur J Pharmacol. 1992 Nov 3;222(1):143–151. doi: 10.1016/0014-2999(92)90826-p. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M., Johnson D. E., Janis R. A., Triggle D. J. Characterization of binding of the ATP-sensitive potassium channel ligand, [3H]glyburide, to neuronal and muscle preparations. J Pharmacol Exp Ther. 1991 Jun;257(3):1162–1171. [PubMed] [Google Scholar]
- Imagawa J., Yoshida S., Koga T., Kamei K., Nabata H. The effect of a novel benzopyran derivative, KC 399, on the isolated guinea-pig trachealis and human bronchi. Gen Pharmacol. 1993 Nov;24(6):1505–1512. doi: 10.1016/0306-3623(93)90444-3. [DOI] [PubMed] [Google Scholar]
- Kajioka S., Nakashima M., Kitamura K., Kuriyama H. Mechanisms of vasodilatation induced by potassium-channel activators. Clin Sci (Lond) 1991 Aug;81(2):129–139. doi: 10.1042/cs0810129. [DOI] [PubMed] [Google Scholar]
- Kamei K., Nabata H., Kuriyama H. Effects of KC 399, a novel ATP-sensitive K+ channel opener, on electrical and mechanical responses in dog tracheal smooth muscle. J Pharmacol Exp Ther. 1994 Jan;268(1):319–327. [PubMed] [Google Scholar]
- Longmore J., Bray K. M., Weston A. H. The contribution of Rb-permeable potassium channels to the relaxant and membrane hyperpolarizing actions of cromakalim, RP49356 and diazoxide in bovine tracheal smooth muscle. Br J Pharmacol. 1991 Apr;102(4):979–985. doi: 10.1111/j.1476-5381.1991.tb12287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K., Okabe K., Kitamura K., Kuriyama H., Weston A. H. Characteristics of cromakalim-induced relaxations in the smooth muscle cells of guinea-pig mesenteric artery and vein. Br J Pharmacol. 1988 Nov;95(3):795–804. doi: 10.1111/j.1476-5381.1988.tb11707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen-Kudsk J. E., Mellemkjaer S., Siggaard C., Nielsen C. B. Effects of pinacidil on guinea-pig airway smooth muscle contracted by asthma mediators. Eur J Pharmacol. 1988 Nov 22;157(2-3):221–226. doi: 10.1016/0014-2999(88)90386-x. [DOI] [PubMed] [Google Scholar]
- Paciorek P. M., Cowlrick I. S., Perkins R. S., Taylor J. C., Wilkinson G. F., Waterfall J. F. Evaluation of the bronchodilator properties of Ro 31-6930, a novel potassium channel opener, in the guinea-pig. Br J Pharmacol. 1990 Jun;100(2):289–294. doi: 10.1111/j.1476-5381.1990.tb15797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast U. Potassium channel openers: pharmacological and clinical aspects. Fundam Clin Pharmacol. 1992;6(7):279–293. doi: 10.1111/j.1472-8206.1992.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Raeburn D., Brown T. J. RP 49356 and cromakalim relax airway smooth muscle in vitro by opening a sulphonylurea-sensitive K+ channel: a comparison with nifedipine. J Pharmacol Exp Ther. 1991 Feb;256(2):480–485. [PubMed] [Google Scholar]
- Schwanstecher M., Brandt C., Behrends S., Schaupp U., Panten U. Effect of MgATP on pinacidil-induced displacement of glibenclamide from the sulphonylurea receptor in a pancreatic beta-cell line and rat cerebral cortex. Br J Pharmacol. 1992 Jun;106(2):295–301. doi: 10.1111/j.1476-5381.1992.tb14331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small R. C., Berry J. L., Burka J. F., Cook S. J., Foster R. W., Green K. A., Murray M. A. Potassium channel activators and bronchial asthma. Clin Exp Allergy. 1992 Jan;22(1):11–18. doi: 10.1111/j.1365-2222.1992.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Arch J. R., Bond J., Buckle D. R., Shaw D. J., Taylor J. F., Ward J. S. The inhibitory effects of cromakalim and its active enantiomer BRL 38227 against various agonists in guinea pig and human airways: comparison with pinacidil and verapamil. J Pharmacol Exp Ther. 1992 May;261(2):429–437. [PubMed] [Google Scholar]
- Xie Z., Hakoda H., Ito Y. Airway epithelial cells regulate membrane potential, neurotransmission and muscle tone of the dog airway smooth muscle. J Physiol. 1992 Apr;449:619–639. doi: 10.1113/jphysiol.1992.sp019105. [DOI] [PMC free article] [PubMed] [Google Scholar]


