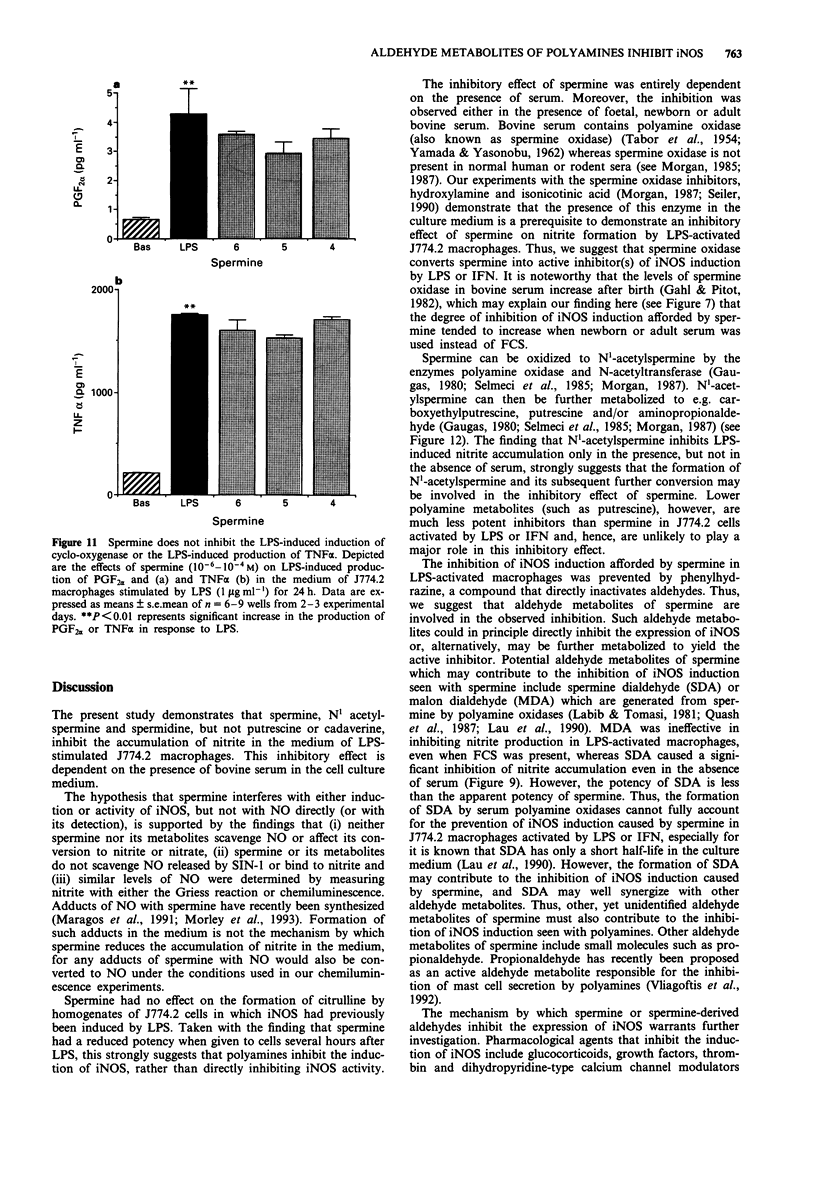
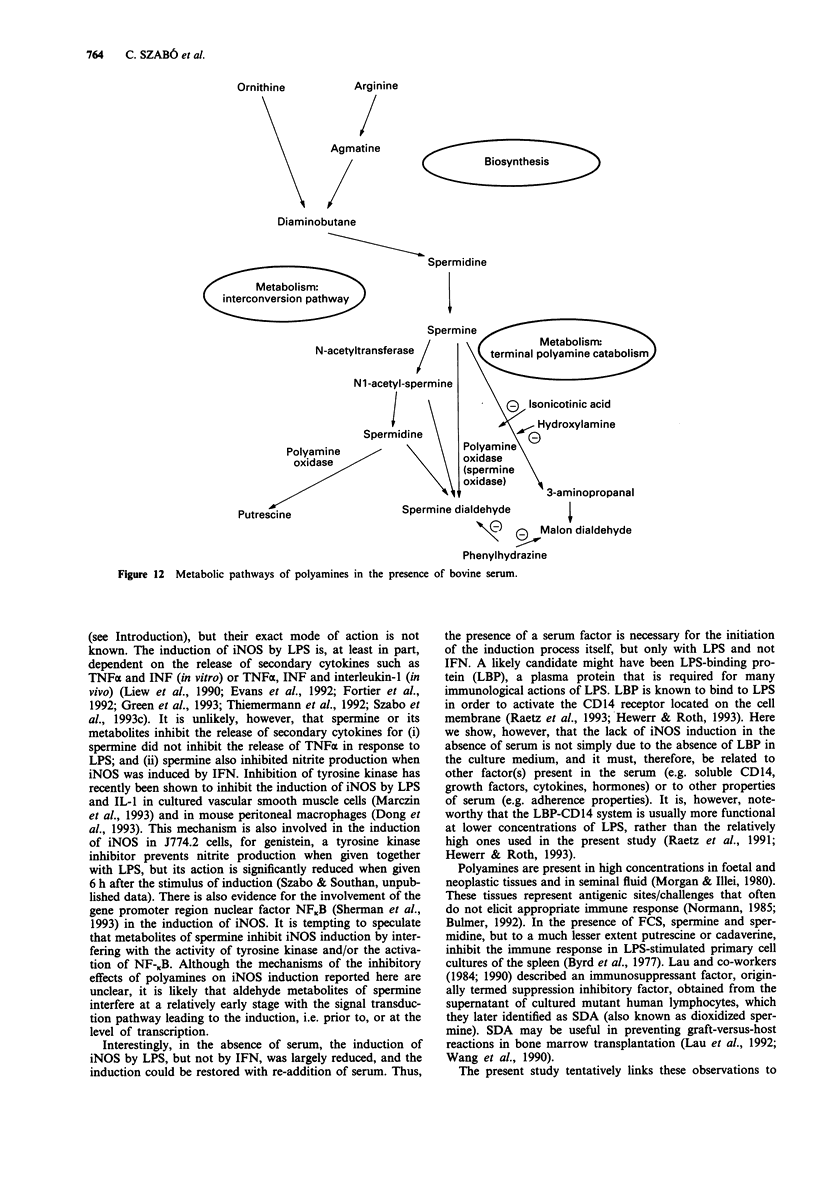
Abstract
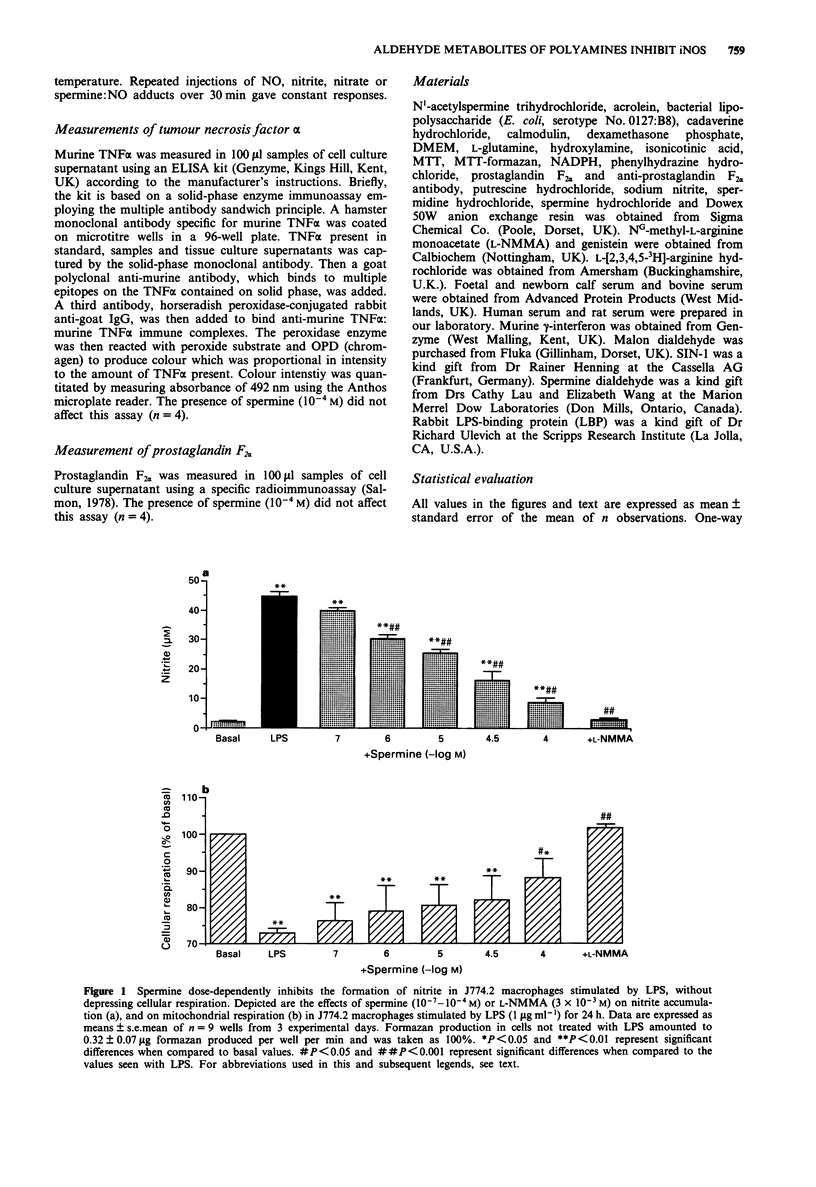
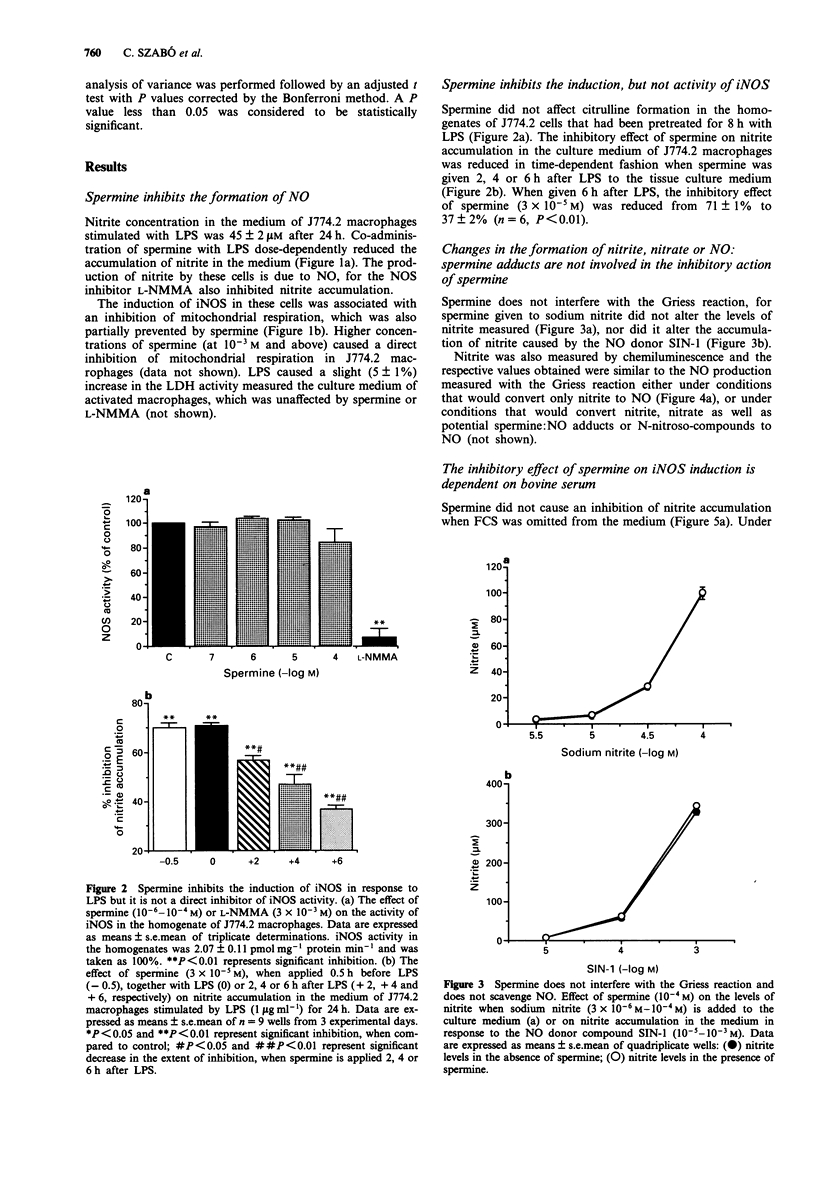
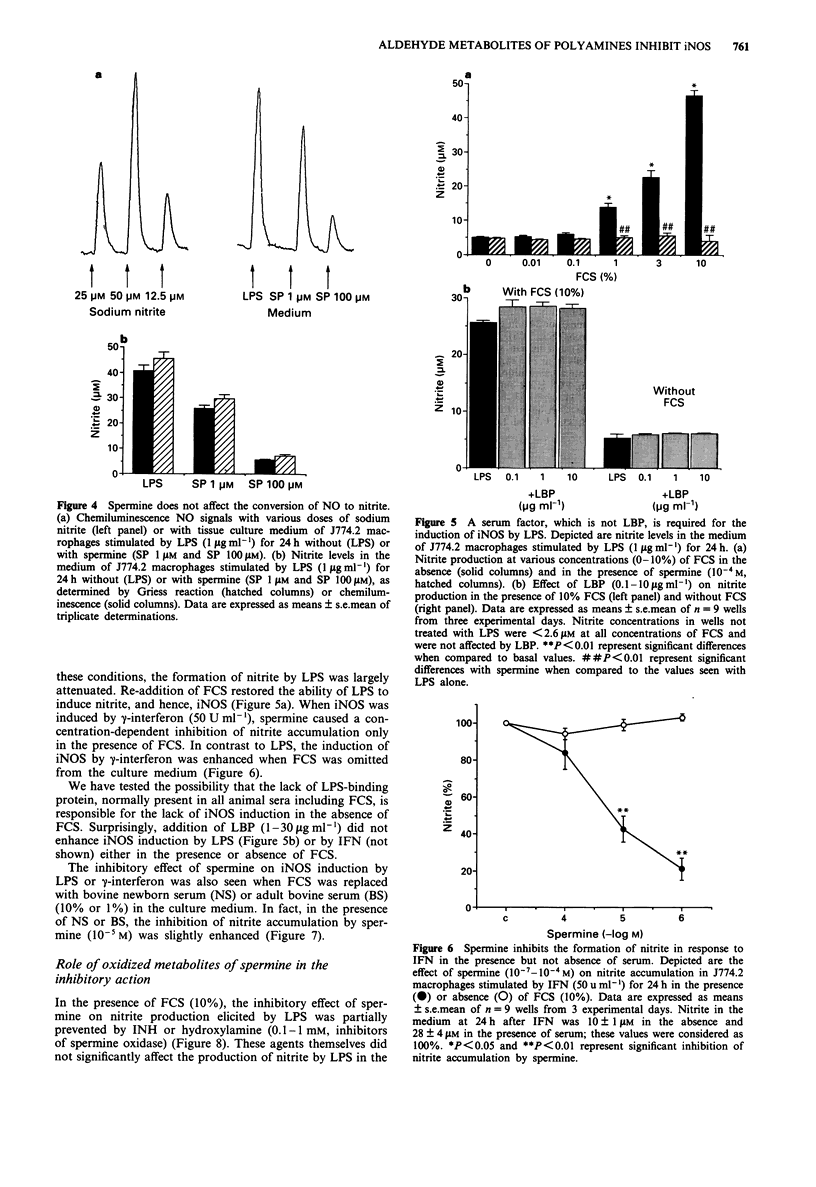
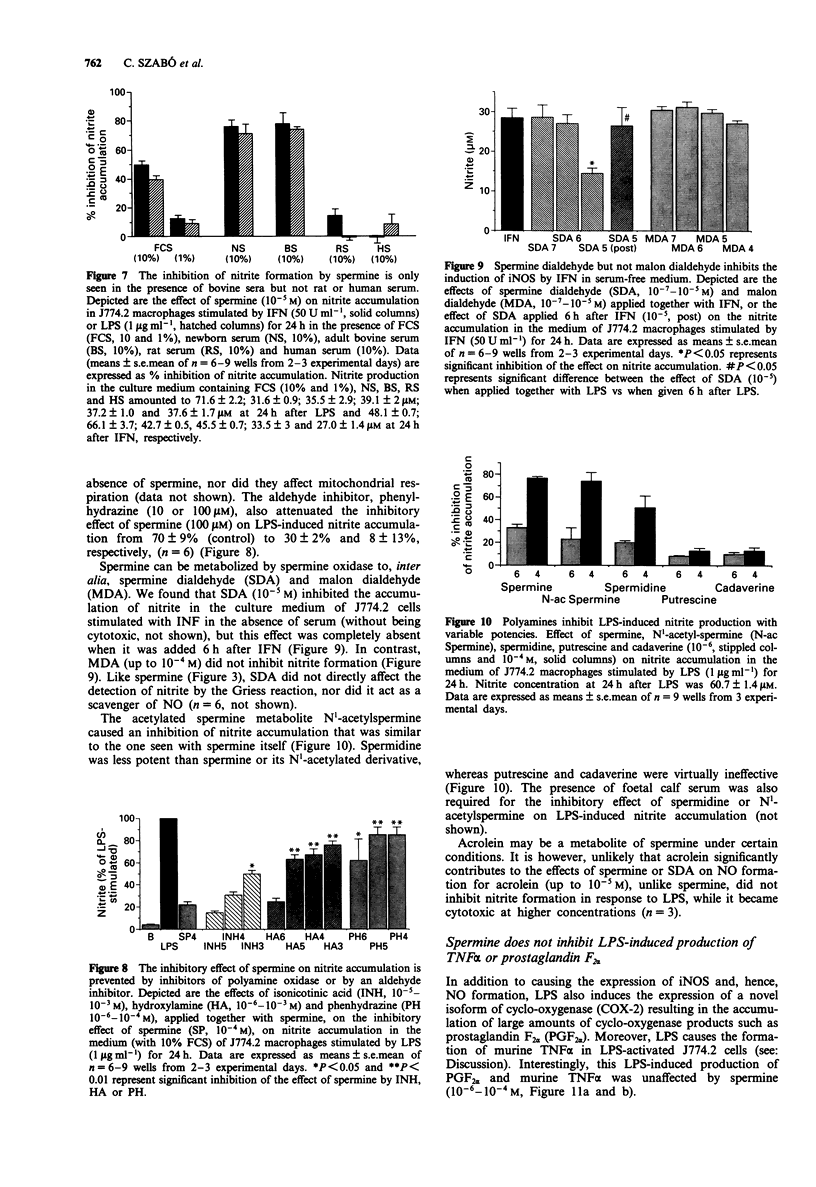
1. We have recently found that in the presence, but not in the absence, of foetal calf serum, spermine inhibits the production of nitric oxide (NO) in cultured J774.2 macrophages stimulated with bacterial endotoxin (lipopolysaccharide; LPS) or with gamma-interferon (IFN), showing that polyamines may act as suppressants of NO-mediated immune functions. Here, we have studied the mechanisms and the specificity of this inhibitory action. 2. Other polyamines, as well as spermine, inhibit the formation of NO in cultured J774.2 macrophages, with the order of potency being spermine > spermidine >> putrescine = cadaverine. This inhibition of NO formation is not due to any cytotoxic effect of these agents for they neither reduced mitochondrial respiration nor increased the release of lactate dehydrogenase into the supernatant. 3. Spermine is not a direct inhibitor of the activity of iNOS in induced J774.2 cells as measured by its lack of effect on the conversion of L-arginine to L-citrulline in homogenates. Neither spermine, nor its metabolites, interfere with the production of nitrite from NO or act as scavengers of NO. Thus, spermine is an inhibitor of the induction of iNOS. 4. Spermine inhibits nitrite formation in the presence of foetal, newborn or adult bovine serum, but not rat or human serum. 5. The effect of sper mine on nitrite production can be prevented by isoniazid, hydrazine or hydroxylamine, inhibitors of spermine oxidase, as well as by phenylhydrazine, an aldehyde inhibitor. We have, therefore, tested the effects of spermine dialdehyde or malon dialdehyde on the induction of iNOS. Spermine dialdehyde (SDA, 10(-5) M) inhibits nitrite formation by IFN-activated J774.2 cells in the absence of serum when given as a pretreatment but not when given 6 h after stimulation. In contrast, malon dialdehyde was ineffective. Thus, aldehyde metabolites of spermine, such as SDA, account for the inhibitory effect of polyamines on the induction of NOS in vitro. 6. The inhibitory effect of polyamines on iNOS induction appears to be fairly specific to iNOS, for spermine does not inhibit LPS-induced production of prostaglandin F2 alpha or tumour necrosis factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bulmer J. N. Immune aspects of pathology of the placental bed contributing to pregnancy pathology. Baillieres Clin Obstet Gynaecol. 1992 Sep;6(3):461–488. doi: 10.1016/s0950-3552(05)80006-9. [DOI] [PubMed] [Google Scholar]
- Byrd W. J., Jacobs D. M., Amoss M. S. Synthetic polyamines added to cultures containing bovine sera reversibly inhibit in vitro parameters of immunity. Nature. 1977 Jun 16;267(5612):621–623. doi: 10.1038/267621a0. [DOI] [PubMed] [Google Scholar]
- Cox R. D., Frank C. W. Determination of nitrate and nitrite in blood and urine by chemiluminescence. J Anal Toxicol. 1982 May-Jun;6(3):148–152. doi: 10.1093/jat/6.3.148. [DOI] [PubMed] [Google Scholar]
- Cunha F. Q., Moncada S., Liew F. Y. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1155–1159. doi: 10.1016/0006-291x(92)91852-h. [DOI] [PubMed] [Google Scholar]
- Dong Z., Qi X., Xie K., Fidler I. J. Protein tyrosine kinase inhibitors decrease induction of nitric oxide synthase activity in lipopolysaccharide-responsive and lipopolysaccharide-nonresponsive murine macrophages. J Immunol. 1993 Sep 1;151(5):2717–2724. [PubMed] [Google Scholar]
- Evans T., Carpenter A., Silva A., Cohen J. Differential effects of monoclonal antibodies to tumor necrosis factor alpha and gamma interferon on induction of hepatic nitric oxide synthase in experimental gram-negative sepsis. Infect Immun. 1992 Oct;60(10):4133–4139. doi: 10.1128/iai.60.10.4133-4139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M., Ostrowski J., Noack E. On the mechanism of NO release from sydnonimines. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S13–S22. [PubMed] [Google Scholar]
- Ferrante A., Maxwell G. M., Rencis V. O., Allison A. C., Morgan D. M. Inhibition of the respiratory burst of human neutrophils by the polyamine oxidase-polyamine system. Int J Immunopharmacol. 1986;8(4):411–417. doi: 10.1016/0192-0561(86)90125-6. [DOI] [PubMed] [Google Scholar]
- Fortier A. H., Polsinelli T., Green S. J., Nacy C. A. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun. 1992 Mar;60(3):817–825. doi: 10.1128/iai.60.3.817-825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahl W. A., Pitot H. C. Polyamine degradation in foetal and adult bovine serum. Biochem J. 1982 Mar 15;202(3):603–611. doi: 10.1042/bj2020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. J., Crawford R. M., Hockmeyer J. T., Meltzer M. S., Nacy C. A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990 Dec 15;145(12):4290–4297. [PubMed] [Google Scholar]
- Green S. J., Nacy C. A., Schreiber R. D., Granger D. L., Crawford R. M., Meltzer M. S., Fortier A. H. Neutralization of gamma interferon and tumor necrosis factor alpha blocks in vivo synthesis of nitrogen oxides from L-arginine and protection against Francisella tularensis infection in Mycobacterium bovis BCG-treated mice. Infect Immun. 1993 Feb;61(2):689–698. doi: 10.1128/iai.61.2.689-698.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett J. A., Roth R. A. Hepatic and extrahepatic pathobiology of bacterial lipopolysaccharides. Pharmacol Rev. 1993 Dec;45(4):382–411. [PubMed] [Google Scholar]
- Labib R. S., Tomasi T. B., Jr Enzymatic oxidation of polyamines. Relationship to immunosuppressive properties. Eur J Immunol. 1981 Mar;11(3):266–269. doi: 10.1002/eji.1830110318. [DOI] [PubMed] [Google Scholar]
- Langrehr J. M., Hoffman R. A., Lancaster J. R., Jr, Simmons R. L. Nitric oxide--a new endogenous immunomodulator. Transplantation. 1993 Jun;55(6):1205–1212. doi: 10.1097/00007890-199306000-00001. [DOI] [PubMed] [Google Scholar]
- Lau C. Y., Budz-Tymkewycz S., Wang E. Y., Ishaque A. A mutant human T-cell line producing immunosuppressive factor(s). Cell Immunol. 1984 Aug;87(1):35–52. doi: 10.1016/0008-8749(84)90128-x. [DOI] [PubMed] [Google Scholar]
- Lau C., Stanojev D., Visconti V., Pang H., Krepinsky G., Grey A., Wang E., Ishaque A. Purification, characterization, and structural elucidation of the active moiety of the previously called "suppressor activating factor (SAF)". Cell Immunol. 1990 Jan;125(1):92–106. doi: 10.1016/0008-8749(90)90065-y. [DOI] [PubMed] [Google Scholar]
- Lau C., Wang E., Ishaque A., Jones K., Wong B., Kimsto L., Conant J. Spermine dialdehyde, a novel ex vivo purging agent for both allogenic and autologous bone marrow transplantations. Int J Immunopharmacol. 1992 Aug;14(6):1081–1091. doi: 10.1016/0192-0561(92)90153-c. [DOI] [PubMed] [Google Scholar]
- Lea R. G., Porat O., Daya S., Fujiwara K., Clark D. A. The detection of spermine and spermidine in human in vitro fertilization supernatants and their relation to early embryo-associated suppressor activity. Fertil Steril. 1991 Oct;56(4):771–775. doi: 10.1016/s0015-0282(16)54614-7. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Tyrphostins: tyrosine kinase blockers as novel antiproliferative agents and dissectors of signal transduction. FASEB J. 1992 Nov;6(14):3275–3282. doi: 10.1096/fasebj.6.14.1426765. [DOI] [PubMed] [Google Scholar]
- Maragos C. M., Morley D., Wink D. A., Dunams T. M., Saavedra J. E., Hoffman A., Bove A. A., Isaac L., Hrabie J. A., Keefer L. K. Complexes of .NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J Med Chem. 1991 Nov;34(11):3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- Marczin N., Papapetropoulos A., Catravas J. D. Tyrosine kinase inhibitors suppress endotoxin- and IL-1 beta-induced NO synthesis in aortic smooth muscle cells. Am J Physiol. 1993 Sep;265(3 Pt 2):H1014–H1018. doi: 10.1152/ajpheart.1993.265.3.H1014. [DOI] [PubMed] [Google Scholar]
- Masferrer J. L., Seibert K., Zweifel B., Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3917–3921. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Seibert K., Needleman P. Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest. 1990 Oct;86(4):1375–1379. doi: 10.1172/JCI114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. M., Illei G. Polyamine-polyamine oxidase interaction: part of maternal protective mechanism against fetal rejection. Br Med J. 1980 May 31;280(6227):1295–1297. doi: 10.1136/bmj.280.6227.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. M. Polyamine oxidases. Biochem Soc Trans. 1985 Apr;13(2):322–326. doi: 10.1042/bst0130322. [DOI] [PubMed] [Google Scholar]
- Morgan D. M. Polyamines. Essays Biochem. 1987;23:82–115. [PubMed] [Google Scholar]
- Morley D., Maragos C. M., Zhang X. Y., Boignon M., Wink D. A., Keefer L. K. Mechanism of vascular relaxation induced by the nitric oxide (NO)/nucleophile complexes, a new class of NO-based vasodilators. J Cardiovasc Pharmacol. 1993 Apr;21(4):670–676. doi: 10.1097/00005344-199304000-00023. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Normann S. J. Macrophage infiltration and tumor progression. Cancer Metastasis Rev. 1985;4(4):277–291. doi: 10.1007/BF00048093. [DOI] [PubMed] [Google Scholar]
- Quan C. P., Roux C., Pillot J., Bouvet J. P. Delineation between T and B suppressive molecules from human seminal plasma: II. Spermine is the major suppressor of T-lymphocytes in vitro. Am J Reprod Immunol. 1990 Jan-Feb;22(1-2):64–69. doi: 10.1111/j.1600-0897.1990.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Quash G., Ripoll H., Gazzolo L., Doutheau A., Saba A., Gore J. Malondialdehyde production from spermine by homogenates of normal and transformed cells. Biochimie. 1987 Feb;69(2):101–108. doi: 10.1016/0300-9084(87)90241-0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Ulevitch R. J., Wright S. D., Sibley C. H., Ding A., Nathan C. F. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J. 1991 Sep;5(12):2652–2660. doi: 10.1096/fasebj.5.12.1916089. [DOI] [PubMed] [Google Scholar]
- Salmon J. A. A radioimmunoassay for 6-keto-prostaglandin F1alpha. Prostaglandins. 1978 Mar;15(3):383–397. doi: 10.1016/0090-6980(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Seiler N. Polyamine metabolism. Digestion. 1990;46 (Suppl 2):319–330. doi: 10.1159/000200405. [DOI] [PubMed] [Google Scholar]
- Sherman M. P., Aeberhard E. E., Wong V. Z., Griscavage J. M., Ignarro L. J. Pyrrolidine dithiocarbamate inhibits induction of nitric oxide synthase activity in rat alveolar macrophages. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1301–1308. doi: 10.1006/bbrc.1993.1359. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Griffith O. W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C., Southan G. J., Wood E., Thiemermann C., Vane J. R. Inhibition by spermine of the induction of nitric oxide synthase in J774.2 macrophages: requirement of a serum factor. Br J Pharmacol. 1994 Jun;112(2):355–356. doi: 10.1111/j.1476-5381.1994.tb13078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C., Thiemermann C., Vane J. R. Dihydropyridine antagonists and agonists of calcium channels inhibit the induction of nitric oxide synthase by endotoxin in cultured macrophages. Biochem Biophys Res Commun. 1993 Oct 29;196(2):825–830. doi: 10.1006/bbrc.1993.2323. [DOI] [PubMed] [Google Scholar]
- Szabó C., Wu C. C., Gross S. S., Thiemermann C., Vane J. R. Interleukin-1 contributes to the induction of nitric oxide synthase by endotoxin in vivo. Eur J Pharmacol. 1993 Nov 30;250(1):157–160. doi: 10.1016/0014-2999(93)90634-t. [DOI] [PubMed] [Google Scholar]
- Szabó C., Wu C. C., Mitchell J. A., Gross S. S., Thiemermann C., Vane J. R. Platelet-activating factor contributes to the induction of nitric oxide synthase by bacterial lipopolysaccharide. Circ Res. 1993 Dec;73(6):991–999. doi: 10.1161/01.res.73.6.991. [DOI] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Tait G. H. Bacterial polyamines, structures and biosynthesis. Biochem Soc Trans. 1985 Apr;13(2):316–318. doi: 10.1042/bst0130316. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Wu C. C., Szabó C., Perretti M., Vane J. R. Role of tumour necrosis factor in the induction of nitric oxide synthase in a rat model of endotoxin shock. Br J Pharmacol. 1993 Sep;110(1):177–182. doi: 10.1111/j.1476-5381.1993.tb13789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliagoftis H., Boucher W. S., Mak L. L., Theoharides T. C. Inhibition of mast cell secretion by oxidation products of natural polyamines. Biochem Pharmacol. 1992 May 28;43(10):2237–2245. doi: 10.1016/0006-2952(92)90183-j. [DOI] [PubMed] [Google Scholar]
- Walters C. L., Gillatt P. N., Palmer R. C., Smith P. L. A rapid method for the determination of nitrate and nitrite by chemiluminescence. Food Addit Contam. 1987 Apr-Jun;4(2):133–140. doi: 10.1080/02652038709373624. [DOI] [PubMed] [Google Scholar]
- Wang E., Conant J. M., Li D., Visconti V., Chourmouzis E., Lau C. Ex vivo spermine dialdehyde treatment prevents lethal GVHD in a murine bone marrow transplantation model. Bone Marrow Transplant. 1990 Oct;6(4):235–242. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. Monoamine oxidase. I. Purification, crystallization, and properties of plasma monoamine oxidase. J Biol Chem. 1962 May;237:1511–1516. [PubMed] [Google Scholar]


