Abstract
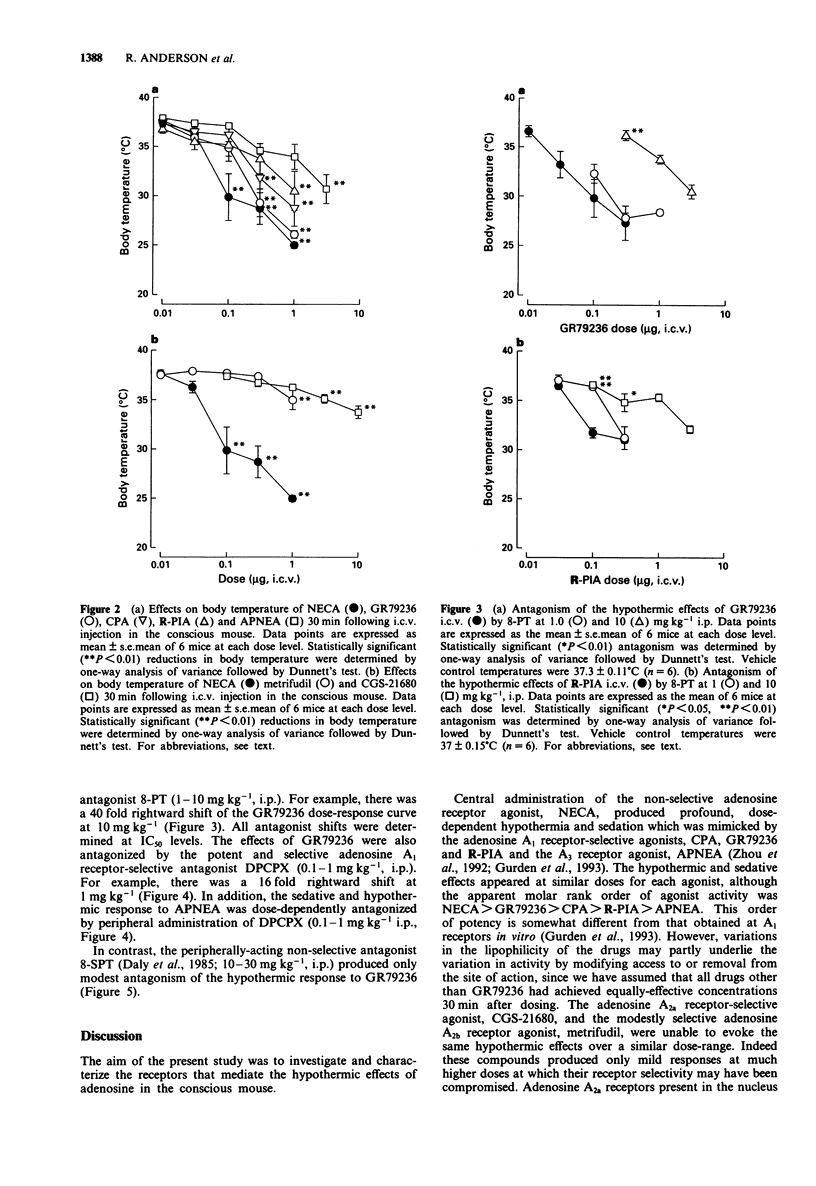
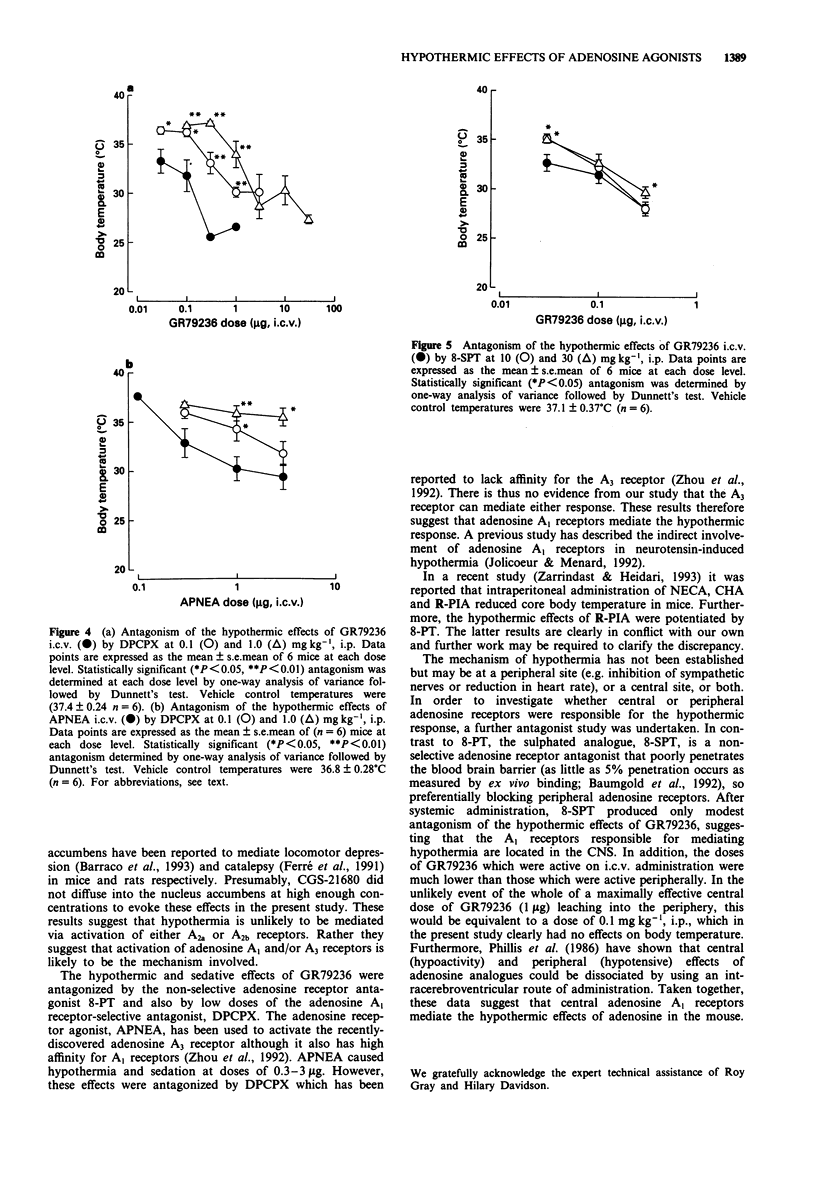
1. The effects of a range of adenosine receptor-selective ligands on body temperature were investigated following intracerebroventricular (i.c.v.) and intraperitoneal (i.p.) injection in conscious mice. The compounds tested were the non-selective adenosine receptor agonist 5'-N-ethyl-carboxamidoadenosine (NECA), the adenosine A1 receptor-selective agonists cyclopentyl-adenosine (CPA), N6-(9R-phenyl-isopropyl)-adenosine (R-PIA) and N-(1S,trans)-[2-hydroxyclopentyl]-adenosine (GR79236), the A2a receptor selective agonist 2-[p-(2-carboxyethyl)phenethylamino]-5'-N-ethylcarboxyamidoaden osine (CGS-21680), the A2b receptor agonist N-[(2-methylphenyl)methyl[adenosine (metrifudil) and the A3 receptor agonist N6-(4-aminophenylethyl)adenosine (APNEA). 2. NECA (0.01-1 microgram, i.c.v.), all of the A1-selective agonists (0.01-1 microgram, i.c.v.) and APNEA (0.1-3 micrograms i.c.v.) produced profound and dose-related hypothermia and sedation. However, CGS-21680 (0.1-10 micrograms i.c.v.) and metrifudil (0.01-1 microgram i.c.v.), produced only mild hypothermia at the highest doses tested. 3. The hypothermic response to the A1 receptor-selective agonists, GR79236 and R-PIA was dose-dependently antagonized by peripheral administration of either the non-selective adenosine receptor antagonist, 8-phenyltheophylline (8-PT, approximately 40 and 30 fold rightward shifts of the dose-response curves respectively at 10 mg kg-1, i.p.), or the adenosine A1 receptor-selective antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, approximately 20 fold shift of the GR79236 dose-response curve at 1 mg kg-1, i.p.). The hypothermic response to APNEA was similarly dose-dependently antagonized by the A1 receptor-selective antagonist, DPCPX (5 fold shift at 0.1 mg kg-1, i.p.).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barraco R. A., Coffin V. L., Altman H. J., Phillis J. W. Central effects of adenosine analogs on locomotor activity in mice and antagonism of caffeine. Brain Res. 1983 Aug 8;272(2):392–395. doi: 10.1016/0006-8993(83)90591-7. [DOI] [PubMed] [Google Scholar]
- Barraco R. A., Martens K. A., Parizon M., Normile H. J. Adenosine A2a receptors in the nucleus accumbens mediate locomotor depression. Brain Res Bull. 1993;31(3-4):397–404. doi: 10.1016/0361-9230(93)90233-2. [DOI] [PubMed] [Google Scholar]
- Baumgold J., Nikodijevic O., Jacobson K. A. Penetration of adenosine antagonists into mouse brain as determined by ex vivo binding. Biochem Pharmacol. 1992 Feb 18;43(4):889–894. doi: 10.1016/0006-2952(92)90257-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W., Padgett W., Shamim M. T., Butts-Lamb P., Waters J. 1,3-Dialkyl-8-(p-sulfophenyl)xanthines: potent water-soluble antagonists for A1- and A2-adenosine receptors. J Med Chem. 1985 Apr;28(4):487–492. doi: 10.1021/jm00382a018. [DOI] [PubMed] [Google Scholar]
- Fastbom J., Pazos A., Probst A., Palacios J. M. Adenosine A1 receptors in the human brain: a quantitative autoradiographic study. Neuroscience. 1987 Sep;22(3):827–839. doi: 10.1016/0306-4522(87)92962-9. [DOI] [PubMed] [Google Scholar]
- Ferré S., Rubio A., Fuxe K. Stimulation of adenosine A2 receptors induces catalepsy. Neurosci Lett. 1991 Sep 16;130(2):162–164. doi: 10.1016/0304-3940(91)90387-9. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Dunwiddie T. V. How does adenosine inhibit transmitter release? Trends Pharmacol Sci. 1988 Apr;9(4):130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol. 1980 Jun 15;29(12):1635–1643. doi: 10.1016/0006-2952(80)90117-3. [DOI] [PubMed] [Google Scholar]
- Gurden M. F., Coates J., Ellis F., Evans B., Foster M., Hornby E., Kennedy I., Martin D. P., Strong P., Vardey C. J. Functional characterization of three adenosine receptor types. Br J Pharmacol. 1993 Jul;109(3):693–698. doi: 10.1111/j.1476-5381.1993.tb13629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison A. J., Williams M., de Jesus R., Yokoyama R., Oei H. H., Ghai G. R., Webb R. L., Zoganas H. C., Stone G. A., Jarvis M. F. 2-(Arylalkylamino)adenosin-5'-uronamides: a new class of highly selective adenosine A2 receptor ligands. J Med Chem. 1990 Jul;33(7):1919–1924. doi: 10.1021/jm00169a015. [DOI] [PubMed] [Google Scholar]
- Jarvis M. F., Williams M. Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective agonist, [3H]CGS 21680. Eur J Pharmacol. 1989 Sep 13;168(2):243–246. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- Jolicoeur F. B., Menard D. Evidence for involvement of A1 adenosine receptors in neurotensin-induced hypothermia. Ann N Y Acad Sci. 1992;668:353–355. doi: 10.1111/j.1749-6632.1992.tb27374.x. [DOI] [PubMed] [Google Scholar]
- Mehta A. K., Kulkarni S. K. Effect of purinergic substances on rectal temperature in mice: involvement of P1-purinoceptors. Arch Int Pharmacodyn Ther. 1983 Aug;264(2):180–186. [PubMed] [Google Scholar]
- Phillis J. W., Barraco R. A., DeLong R. E., Washington D. O. Behavioral characteristics of centrally administered adenosine analogs. Pharmacol Biochem Behav. 1986 Feb;24(2):263–270. doi: 10.1016/0091-3057(86)90349-7. [DOI] [PubMed] [Google Scholar]
- Yarbrough G. G., McGuffin-Clineschmidt J. C. In vivo behavioral assessment of central nervous system purinergic receptors. Eur J Pharmacol. 1981 Dec 3;76(2-3):137–144. doi: 10.1016/0014-2999(81)90495-7. [DOI] [PubMed] [Google Scholar]
- Zhou Q. Y., Li C., Olah M. E., Johnson R. A., Stiles G. L., Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]


