Abstract
Nitrogen-containing bisphosphonates were shown to cause macrophage apoptosis by inhibiting enzymes in the biosynthetic pathway leading from mevalonate to cholesterol. This study suggests that, in osteoclasts, geranylgeranyl diphosphate, the substrate for prenylation of most GTP binding proteins, is likely to be the crucial intermediate affected by these bisphosphonates. We report that murine osteoclast formation in culture is inhibited by both lovastatin, an inhibitor of hydroxymethylglutaryl CoA reductase, and alendronate. Lovastatin effects are blocked fully by mevalonate and less effectively by geranylgeraniol whereas alendronate effects are blocked partially by mevalonate and more effectively by geranylgeraniol. Alendronate inhibition of bone resorption in mouse calvaria also is blocked by mevalonate whereas clodronate inhibition is not. Furthermore, rabbit osteoclast formation and activity also are inhibited by lovastatin and alendronate. The lovastatin effects are prevented by mevalonate or geranylgeraniol, and alendronate effects are prevented by geranylgeraniol. Farnesol and squalene are without effect. Signaling studies show that lovastatin and alendronate activate in purified osteoclasts a 34-kDa kinase. Lovastatin-mediated activation is blocked by mevalonate and geranylgeraniol whereas alendronate activation is blocked by geranylgeraniol. Together, these findings support the hypothesis that alendronate, acting directly on osteoclasts, inhibits a rate-limiting step in the cholesterol biosynthesis pathway, essential for osteoclast function. This inhibition is prevented by exogenous geranylgeraniol, probably required for prenylation of GTP binding proteins that control cytoskeletal reorganization, vesicular fusion, and apoptosis, processes involved in osteoclast activation and survival.
Keywords: isoprenylation, geranylgeranylation, statin, bisphosphonate, osteoporosis
Bisphosphonate (BP) suppression of bone resorption has been known for over 30 years. It is now generally accepted that BPs inhibit osteoclast (Oc) activity; however, there is still limited understanding of the mode of action of BPs, especially at the molecular level (1). In vitro evidence suggests that BPs inhibit Oc recruitment by acting on osteoblast lineage cells (2). In vitro and in vivo studies have shown that BPs can increase Oc apoptosis (3). However, it is not known whether apoptosis can fully account for BP inhibition of bone resorption and whether BPs activate an apoptotic pathway directly or whether apoptosis is secondary to Oc inactivation.
The object of this study is to examine whether BPs act on the Oc directly and to identify their putative molecular target in these cells. Structure–activity relationship investigations of BP inhibition of bone resorption (4), of cell growth inhibition in slime molds (5), and of macrophage apoptosis (6, 7) support the assumption that nitrogen-containing BPs interact with a highly specific target inside these cells rather than causing a nonspecific toxic effect. Given the BP structure, these molecules are putative inhibitors of intracellular pathways involving phosphate or pyrophosphate. It was shown that BPs can inhibit various tyrosine phosphatases (8); however, none of the phosphatases tested showed rank order sensitivity to the clinically used BPs that corresponded to their potency for inhibition of bone resorption. Several years ago, it was reported that BPs inhibit squalene synthase, which uses farnesyl diphosphate (FPP) as a substrate (9). The rank order of potency for squalene synthase inhibition correlated with that for inhibition of bone resorption, suggesting the possibility that interference with this pathway may be related to Oc inhibition. Based on this hypothesis, Luckman et al. (7) evaluated the effect of mevastatin, an inhibitor of hydroxymethylglutaryl CoA reductase in the cholesterol biosynthesis pathway, on a mouse monocyte/macrophage cell line, J774. This cell line was chosen as a surrogate for Ocs because the two cell types are derived from a common progenitor and both undergo apoptosis after treatment with BPs (3, 6). Mevastatin, like alendronate (ALN), induced apoptosis in this cell line, and these effects were prevented by mevalonate, FPP, or geranylgeranyl diphosphate (GGPP). These findings suggested that ALN and probably other N-containing BPs inhibit steps in the cholesterol biosynthesis pathway, which can be compensated for by the exogenous addition of the affected metabolites. FPP and GGPP are downstream of mevalonate in this pathway and are important substrates in the prenylation of many proteins involved in cytoskeletal function and vesicular trafficking, including Rho, Rac, Cdc42, and Rab (10, 11). Cytoskeletal function clearly is disrupted in Ocs after ALN treatment, as evidenced by the disappearance of the ruffled border (12). The findings of this study support the hypothesis that ALN inhibition of enzymes in the cholesterol metabolic pathway, most likely those responsible for the generation or utilization of GGPP but not FPP, plays an important role in its effect on Ocs. Direct action of ALN on purified Ocs is supported by the observation of ALN stimulation of a 34-kDa kinase. This kinase response is blocked by all-trans geranylgeraniol (GGOH) but not farnesol (FOH), suggesting mechanism-based activation.
MATERIALS AND METHODS
Murine Osteoclastogenesis Assay.
Murine cocultures of osteoblasts and marrow cells were prepared by using the methods of Wesolowski et al. (13). Bone marrow cells were harvested from 6-week-old male BALB/c mice by flushing the marrow spaces of freshly isolated long bones (tibiae and femora) with α-MEM (minimal essential media) containing penicillin/streptomycin (100 units and 100 μg/ml, respectively) and 20 mM Hepes buffer. Bone marrow cells were suspended in α-MEM supplemented with fetal calf serum (10% vol/vol) and 10 nM 1,25-(OH)2 vitamin D3. Bone marrow cells then were added to subconfluent monolayers of osteoblastic MB1.8 cells in 24-well cell culture plates in quadruplicate wells and were cultured for 5 days at 37°C in the presence of 5% CO2. Stock solutions of FOH, squalene, and GGOH were added to cultures on days 5 and 6. On day 7, the cultures were evaluated by counting the number of tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells, as described below.
TRAP Staining.
Cells were fixed with 10% formalin in PBS and were rinsed with Hepes-buffered solution (0.9% NaCl/10 mM Hepes, pH 7.1). Cells then were stained with Fast Red Violet LB dissolved in TRAP buffer (50 mM sodium acetate/30 mM sodium tartrate/0.1% Triton X-100/100 μg Naphthol AS-MX phosphate, pH 5.0) for 10 min at 37°C. Oc-like cells (Ocls) (i.e., TRAP positive cells ≥250 μm in diameter) in each well were quantitated microscopically by using a Nikon Diaphot microscope equipped with a 10× objective.
Assessment of Osteoclast Number and Bone Resorption in Isolated Murine Calvaria.
Osteoclast number and bone resorption were assessed as described (7,14). In brief, calvariae were dissected from 1-day-old mice, cut into halves, and cultured in 24-well plates for 24 hr. Fresh medium containing 10−8 M parathyroid hormone then was added, and the calvariae were incubated for a further 24 hr in the absence or presence of 1 mM MVA, 10 μM ALN, 10 μM ALN and 1 mM MVA, 100 μM clodronate (CLOD), and 100 μM CLOD and 1 mM MVA. The calvariae then were washed with PBS and were incubated for 30 min with nitroblue tetrazolium (2 mg/ml) in PBS, were washed with PBS, and finally were fixed with 4% paraformaldehyde in PBS. Osteoclasts (stained with insoluble blue formazan, which is formed after addition of the nitroblue tetrazolium) were counted over the entire area of the parietal bones, and resorption (areas of increased lucency) was estimated by point counting using an eyepiece graticule, sampling the most central field of the parietal bones at 63× magnification.
Rabbit Osteoclast Bone Resorption Assay.
Tibiae were aseptically isolated from 10-day-old New Zealand White rabbits (Covance Research Products, Denver, PA), and the soft tissue was removed. Bones were minced into 1-mm pieces in α-MEM containing Hepes (10 mM, pH 7.1) and penicillin/streptomycin (100 units and 100 μg/ml, respectively). Bone fragments were rocked gently for 60 cycles, and tissue was allowed to settle. Supernatants were supplemented with fetal bovine serum (HyClone) to 10%. Cells (106/ml in α-MEM containing 10% fetal bovine serum) were plated onto bovine bone slices (6-mm diameter × 200-μm thickness). After 1 hr, test compounds were diluted in α-MEM containing 10% fetal bovine serum, 10 nM 1,25(OH)2 vitamin D3 and were added into triplicate wells. Cultures were incubated for 3 days at 37°C in a 5% CO2 atmosphere. Collagen fragments released into the medium were measured by the CrossLaps ELISA assay (Osteometer Biotech, Herlev, Denmark).
Ocl Purification, Treatment, and Lysate Formation.
Ocls were isolated from murine bone marrow/MB1.8 osteoblast cocultures as described above. Cocultures first were treated with collagenase (Wako Pure Chemical, Osaka) at a concentration of 1 mg/ml (in PBS) for 1 hr at 37°C to remove osteoblasts and stromal cells. Resistant cells (primarily prefusion-Ocs and Ocls) then were treated with EDTA (0.2 g/l in PBS) for 20 min at 37°C to remove prefusion-Ocs. Ocls (90–95% purity) were maintained in α-MEM supplemented with fetal bovine serum (10%), penicillin/streptomycin (100 units and 100 μg/ml, respectively), 1,25 (OH)2 vitamin D3 (10 nM), and macrophage-colony stimulating factor (5 ng/ml). Ocls were treated with indicated compounds in the above-mentioned medium for indicated times. After treatments, cells were placed on ice and washed with ice-cold β-glycerophosphate-Hepes-buffered solution: 50 mM Hepes (pH 7.6), 50 mM β-glycerophosphate, 1 mM EDTA, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. Ocls then were lysed in β-glycerophosphate-Hepes-buffered solution containing the following additions: 0.2% Triton X-100, 1 μM Microcystin LR, 1 mM Na3VO4, 1 mM DTT, and 1 mM phenylmethylsulfonyl fluoride. Protein concentrations were determined by using a Bradford reagent kit (Bio-Rad).
In-Gel Kinase Assay.
Kinase assays were performed in the gel by using methods based on Kameshita and Fujisawa (15) and Gotoh et al. (16). In brief, 10% SDS/PAGE gels were cast with myelin basic protein (0.1–0.2 mg/ml) added before polymerization. Lanes were loaded with equivalent amounts of protein lysate (5–10 μg/lane) and were electrophoresed. Gels were washed twice at room temperature with buffer A: 50 mM Hepes (pH 7.6), 5 mM 2-mercaptoethanol supplemented with isopropanol (20%), followed by two washes with buffer A. Proteins were denatured with urea (6 M in buffer A) at room temperature and were renatured at 4°C with urea at 3 M and then 0.75 M (in buffer A), followed by three washes with buffer A containing Tween 20 (0.05%). Gels then were washed twice with kinase buffer (20 mM Hepes, pH 7.6/20 mM MgCl2/2 mM DTT) at 30°C. Kinase reactions lasted 30 min at 30°C using kinase buffer containing 0.02 mM ATP with ≈1,000 cpm/pmol 32P-γ-ATP. Kinase reactions were stopped, and unincorporated 32P-γ-ATP was removed with six washes by using 5% trichloroacetic acid/1% NaPPi (wt/vol). Gels then were stained, destained, and dried by using standard techniques. Kinase activities were visualized by autoradiography.
RESULTS AND DISCUSSION
Several BPs inhibit squalene synthase with a potency rank that resembles that for inhibition of bone resorption (9). Recently, it was shown that ALN induces apoptosis in the mouse macrophage cell line J774; this effect was similar to that of mevastatin and was blocked by the presence of FPP or GGPP, suggesting that ALN inhibits one or several steps in the cholesterol biosynthesis pathway (7). To further investigate this possibility, we examined the effect of cholesterol biosynthesis pathway intermediates on ALN inhibition of Oc generation and of bone resorption in several in vitro systems: (i) Oc generation from mouse bone marrow; (ii) Oc formation and bone resorption in mouse calvaria; and (iii) the formation of rabbit Ocs and their activity in a bone slice “pit” assay in culture. (iv) We also examined the effect of GGOH on ALN stimulation of a kinase in Ocs.
Effect of Cholesterol Pathway Intermediates on Lovastatin and Alendronate Inhibition of Osteoclast Formation.
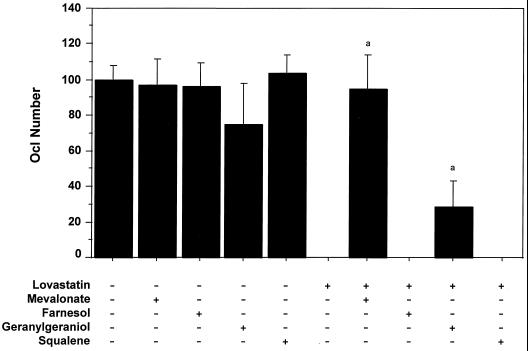
Ocls are formed in vitro from mouse bone marrow cultured with the osteoblastic cells (MB1.8 cell line) in the presence of 1,25-(OH)2 vitamin D3 (10−8 M). TRAP-positive cells, produced in this coculture, resorb bone and have a large number of calcitonin and αvβ3 integrin receptors (13). Addition of 10 μM lovastatin (LOV), a hydroxymethylglutaryl CoA reductase inhibitor, during the last 48 hr of osteoclastogenesis (starting at day 5 of the coculture) completely eliminated large (≥250 μm) Ocls from the culture (Fig. 1). LOV treatment did not induce cell death or changes in morphology of MB1.8 cells (data not shown), suggesting that broad cell toxicity was not responsible for the decline in Ocl number. Thus, effects on Ocl formation could be attributable to inhibition of differentiation/fusion and/or induction of apoptosis. Because inhibition of hydroxymethylglutaryl CoA reductase by LOV prevents the conversion of β-OH-β-methylglutaryl-CoA to mevalonate, this suggests that mevalonate or one of its downstream metabolites is required for Oc generation. To test this hypothesis, metabolites of the pathway were added along with 10 μM LOV. Mevalonic acid lactone (MVA) (1 mM) completely restored LOV-inhibited osteoclastogenesis, indicating that the LOV effect was caused by inhibition of mevalonate formation (Fig. 1) and not by nonspecific cell toxicity. Three metabolites, downstream of mevalonate, were also examined. FOH (10 μM) and squalene (10 μM) were without effect; however, addition of GGOH (10 μM), which is metabolized to GGPP in the cells (reviewed in ref. 17), prevented the complete block in formation of Ocls produced by LOV, resulting in Ocl formation to levels 30% of untreated controls (Fig. 1). This suggested that inhibition of geranylgeranylation was an important component of LOV inhibition of Oc formation.
Figure 1.
Lovastatin inhibits the formation of osteoclasts in vitro but not in the presence of mevalonate or geranylgeraniol. Osteoclastogenesis was assessed by using a coculture of mouse bone marrow cells and MB1.8 osteoblast cells as described in the Materials and Methods. On days 5 and 6 of the coculture, 10 μM LOV was added in the absence (−) or presence (+) of the following cholesterol biosynthesis metabolites: 1 mM MVA, 10 μM FOH, 10 μM GGOH, and 10 μM squalene. Osteoclast number was scored on day 7 by assessing numbers of large (i.e., ≥250 μm) TRAP-positive multinucleate cells (mean ± SD, n ≥ 3). a, statistically significantly different from LOV (P < 0.0001).
GGPP is primarily involved in the prenylation of GTP binding proteins such as Rac, Rho, cdc42, and Rab (11), which are implicated in cytoskeletal function and vesicular trafficking. Oc activation involves substantial cytoskeletal reorganization that leads to cellular polarization and the formation of the actin-rich attachment membrane called “clear zone” and the ruffled border. After BP treatment, osteoclasts lack the ruffled border (12), which could be because of interference with the cytoskeletal reorganization required for osteoclast polarization. Rab, in particular, has been implicated in vesicular fusion (18), which could be a mechanism for ruffled border generation (reviewed in ref. 19).
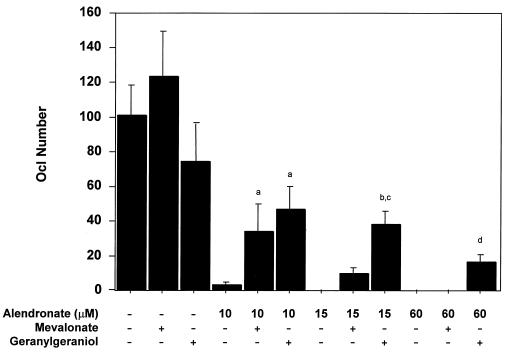
The next experiments examined in the same system the effect of MVA and GGOH on ALN inhibition of osteoclast formation. As shown in Fig. 2, both metabolites suppressed ALN inhibition of osteoclastogenesis. GGOH (10 μM) was more effective than MVA (1 mM), and suppression decreased with increasing ALN concentration. This raises the possibility of ALN inhibition of MVA conversion to GGPP and/or competition with GGPP in a downstream reaction. ALN inhibition of several enzymes with diphosphate-containing substrates is certainly possible. Addition of 10 μM farnesol or 10 μM squalene to this system had no effect on ALN inhibition (data not shown).
Figure 2.
Geranylgeraniol and, to a lesser extent, mevalonate blocks alendronate inhibition of osteoclast formation in vitro. Osteoclastogenesis cultures were performed as described in Fig. 1 in the absence or presence of ALN at 10, 15, and 60 μM doses. MVA (1 mM) or GGOH (10 μM) were added to the above cocultures, and TRAP-positive fully mature (i.e., ≥250 μm) multinucleate Ocls were quantitated microscopically (mean ± SD). a, statistically significantly different from ALN (10 μM) (P < 0.0001); b, statistically significantly different from ALN (15 μM) (P < 0.0001); c, statistically significantly different from ALN (15 μM) + MVA (1 mM) (P < 0.0001); d, statistically significantly different from ALN (60 μM) (P < 0.02).
Effect of Cholesterol Pathway Intermediates on ALN Inhibition of Murine Osteoclast Formation and Bone Resorption in Murine Calvaria.
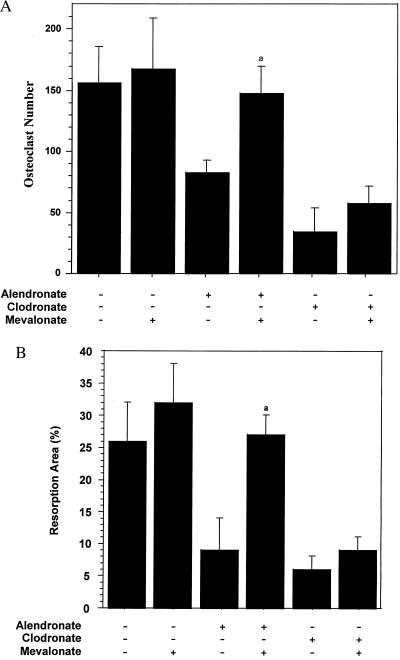
The following experiments were designed to examine the effects of MVA on suppression of Oc number and function in mouse calvaria explants. In this study, the action of ALN was compared with that of the BP, clodronate, which does not contain a nitrogen and is suggested to act via a different mechanism, the formation of a cytotoxic metabolite (20). Mouse calvaria from 1-day-old mice were explanted, halved, and cultured in the presence of parathyroid hormone. These were treated with 10 μM ALN or 100 μM CLOD in the absence or presence of 1 mM MVA, as described in Materials and Methods. Treatment with either ALN or CLOD resulted in a 50–75% reduction in the number of osteoclasts, respectively (Fig. 3A). Identical results were obtained when the Ocs were stained for TRAP (data not shown). Similarly, a 60–70% reduction in bone resorption was observed (Fig. 3B). Co-incubation of MVA with ALN resulted in full recovery of Oc number, as well as bone resorption. Like in the system described above, MVA opposed ALN inhibition. The larger effect of MVA in this system may reflect differences in the biology of osteoclastic resorption in calvaria, relative to isolated osteoclasts from mouse bone marrow. In addition, in calvaria, there is substantial uptake of ALN by the bone surface, which makes it difficult to estimate the actual exposure of Ocs to ALN. Also, parathyroid hormone was used to stimulate resorption. In contrast to the suppression of ALN effects, MVA was unable to significantly rescue the number and function of osteoclasts after CLOD treatment, supporting the concept of a different mechanism of action for this BP (20).
Figure 3.
Mevalonate blocks alendronate, but not clodronate, inhibition of osteoclast number and bone resorption in mouse calvaria. Calvariae from 1-day-old mice were halved and cultured for 24 hr in media containing parathyroid hormone and either ALN (10 μM) or CLOD (100 μM) in the presence or absence of MVA (1 mM) as described in Materials and Methods. Osteoclasts were scored over the entire area of the parietal bones after staining with nitroblue tetrazolium (A), and bone resorption was scored by increased lucency within the central region of the parietal bones (B) (mean ± SD, n ≥ 3). a, statistically significantly different from ALN (P < 0.01).
Effect of Cholesterol Pathway Intermediates on ALN Inhibition of Rabbit Osteoclastogenesis and Bone Resorption.
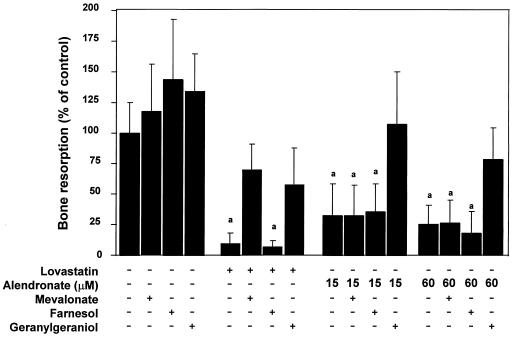
The same questions were addressed by following the formation of TRAP(+) multinucleated cells from rabbit tibia. The rabbit cells plated on tissue culture plastic dishes, as described in Materials and Methods, underwent cell fusion over a period of 3 days. Treatment of these cultures with ALN at 15 μM and 60 μM resulted in ≈50% reduction in the number of fused TRAP(+) cells (data not shown). Microscopic examination showed that the surviving cells were retracted and vacuolated. In this system, GGOH restored the number of fused cells to levels obtained in the absence of ALN. MVA was without effect (data not shown). In parallel, we examined GGOH effects on LOV and ALN inhibition of bone resorption, estimated by measuring a collagen degradation product, C-telopeptide, in the media (Fig. 4). LOV at 10 μM inhibited bone resorption by 90% in this system, and this effect was significantly opposed by MVA (1 mM) and to a similar extent by GGOH (10 μM). ALN (15 and 60 μM) inhibited bone resorption by 70–80% (Fig. 4). GGOH, but not MEV, fully blocked this inhibition, which was similar to its effect on ALN inhibition of osteoclastogenesis from mouse bone marrow, suggesting a similarity between the action of ALN and LOV in this system. In both cases, FOH (10 μM) was without effect.
Figure 4.
Effects of cholesterol biosynthesis metabolites on inhibition of rabbit osteoclast-mediated bone resorption in vitro. Rabbit osteoclasts were isolated from the tibiae and femora of 10-day-old New Zealand White rabbits and then were plated onto bovine bone slices (6-mm diameter × 200-μm thickness). LOV (10 μM) or ALN (15 or 60 μM) were added in the absence or presence of the following: MVA (1 mM), FOH (10 μM), and GGOH (10 μM). After 3 days incubation, collagen fragments released into the medium were measured by the CROSSLAPS ELISA assay as described in the Materials and Methods. (mean ± SD, n = 3) a, statistically significantly different from control (P < 0.0001).
Taken together, these findings suggest that GGOH, converted to GGPP, replaces the cholesterol biosynthetic pathway intermediate whose suppression accounts for some or all of the inhibitory effects of ALN. This could not be deduced from the study of the J774 macrophage/monocyte cell line, in which either FPP or GGPP could block ALN-induced apoptosis (7). The lack of effect of MVA in some of the Oc systems is consistent with the involvement of an enzyme downstream of mevalonate, probably one necessary for the formation of GGPP. ALN could interfere with the generation of GGPP, possibly by competing with the FPP or isopentenyl diphosphate at one or several enzymatic steps in the pathway. The role of GGPP in the prenylation of Rac, Cdc42, Rho, or Rab could be responsible for the effects observed. The data of Luckman et al. (7), showing reduced protein prenylation in J774 macrophages treated with ALN, which causes apoptosis in these cells, is consistent with this hypothesis. Independent effects of GGOH on Oc survival and function cannot be excluded at this time. It also remains to be shown whether ALN directly inhibits the enzyme(s) responsible for generation of GGPP or affects its function as a substrate at concentrations of ALN that may be present in the cell. Previous studies have estimated that concentrations of ALN as high as 300 μM can be generated in the lacunae under osteoclasts after acidification of the ALN-containing bone surface during the initial stages of Oc activity (12). A likely mechanism for BP uptake in the Oc is the recently described transcytotic process that transfers resorption products from the extracellular resorption domain to the basolateral surface (21). ALN (up to 100 μM) in vitro did not inhibit geranylgeranyl transferases I or II (H. Huber, personal communication), suggesting that the abundance rather than the transfer of GGPP may be affected.
GGOH Suppression of ALN Activation of a 34-kDa Kinase in Murine Osteoclasts.
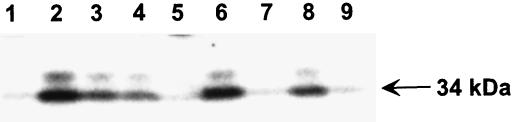
The cytoskeletal changes associated with ALN inhibition of Oc activity, whether induced by inhibition of tyrosine phosphatases (8) or prenylation of G proteins, as suggested above, should have downstream effects on signal transduction pathways and on various kinases. To examine some of these downstream events, we used an in-gel kinase assay (15, 16), which evaluates changes in the activity of a broad spectrum of kinases. Kinase activity was examined in lysates of 90–95% pure murine Ocls (isolated as described in Materials and Methods). Mature Ocls treated for 17–24 hrs with ALN (30 μM) showed a 7-fold increase in the activity of a 34-kDa kinase (Fig. 5, lane 2), relative to untreated controls (Fig. 5, lane 1). Similarly, 10 μM LOV treatment induced this kinase to an equivalent extent (Fig. 5, lane 6). A 36-kDa kinase showed a similar but weaker response to either ALN or LOV treatment. Most interestingly, kinase activation by ALN or LOV was blocked by the presence of 10 μM GGOH (Fig. 5, lanes 5 and 9), consistent with GGOH suppression of ALN inhibition of Oc activity. MVA completely blocked the LOV effects (Fig. 5, lane 7) but was not effective against ALN stimulation (Fig. 5, lane 3). Farnesol had little effect on 34-kDa kinase activation by either ALN or LOV (Fig. 5, lanes 4 and 8, respectively). These findings are strikingly similar to our observations that MVA and GGOH suppress ALN and LOV inhibition of Oc formation and bone resorption. Two other N-containing BPs, pamidronate and risedronate, used at 10–100 μM, also activate the 34-kDa kinase whereas etidronate and tiludronate, which do not contain N-groups, failed to activate this kinase at up to 300 μM (A.A.R., P.J.M., and G.A.R., unpublished work), supporting different molecular mechanisms for the two groups of BPs. This identifies an intracellular molecular change in isolated Ocs treated with ALN. Based on 90–95% purity of the Ocls, this suggests direct effects on the Oc.
Figure 5.
Activation of a 34-kDa kinase by alendronate and lovastatin is blocked by geranylgeraniol and, to a lesser extent, mevalonate. Ocls were purified from cocultures (see Fig. 1) by sequential treatment of culture dishes with collagenase and then EDTA, as described in Materials and Methods. Ocls then were left untreated (lane 1) or were incubated with ALN (30 μM; lanes 2–5) or LOV (10 μM; lanes 6–9) in the presence of MVA (1 mM; lanes 3 and 7), FOH (10 μM; lanes 4 and 8), or GGOH (10 μM; lanes 5 and 9) for 17 hr. Cell lysates were analyzed by in-gel kinase assay using myelin basic protein as a substrate. Activity of 34-kDa (arrow) and 36-kDa kinases was visualized by autoradiography. Data is representative of three independent experiments.
Direct action of BPs on Ocs is also supported by the preferential localization of BPs beneath Ocs in vivo followed by BP uptake (as described above) and disappearance of the ruffled border (12, 22), which is indicative of Oc inactivation. The detection of the bisphosphonate, ALN, inside the Oc (22) suggests an intracellular target, consistent with the observations of Murakami et al. (23) showing that disruption of the actin ring produced by BPs in normal Ocs is not seen in Ocs from the genetically defective oc/oc mice, unless the BP is microinjected into these resorption-deficient Ocs (23). Intracellular action is also consistent with the effect of BPs on Dictyostelium discoideum, which requires pinocytotic uptake of BP to inhibit its growth (24). Luckman et al. (7) have shown that ALN blocks de novo prenylation of Ras in J774 cells. We also have observed inhibition of Ras prenylation by ALN in Ocls (data not shown). ALN effects on prenylation of other GTPases is under investigation.
In conclusion, the data presented here support direct action of ALN on Ocs by the following mechanism. After administration, ALN binds to the bone mineral and then is taken up by osteoclasts during initiation of resorption. Inside the osteoclasts, it interferes with the generation of GGPP by inhibition of an enzyme(s) in the cholesterol biosynthesis pathway, resulting, most likely, in a loss of prenylation of GTP-binding proteins that control cytoskeletal function, vesicular trafficking, and apoptosis. The target of ALN action is likely to be an enzyme involved in the conversion of mevalonate to GGPP, such as isopentenyl diphosphate/dimethylallyl diphosphate isomerase, FPP synthase, or GGPP synthase, which use diphosphate substrates. The result is disruption of vesicular fusion and of ruffled border formation, possibly via downstream kinases. This investigation identified a 34-kDa kinase regulated in the osteoclast by BP treatment. Its characterization is the subject of further studies.
Acknowledgments
The authors thank Rose Nagy for technical assistance and Dianne McDonald for preparation of the manuscript. The research contributed by J.E.F. is in partial fulfillment of his Ph.D. thesis dissertation.
ABBREVIATIONS
- BP
biphosphonate
- Oc
osteoclast
- FPP
farnesyl diphosphate
- ALN
alendronate
- GGPP
geranylgeranyl diphosphate
- GGOH
geranylgeraniol
- FOH
farnesol
- TRAP
tartrate-resistant acid phosphatase
- CLOD
clodronate
- Ocl
Oc-like cell
- LOV
lovastatin
- MVA
mevalonic acid lactone
References
- 1.Rodan G A, Fleisch H A. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahni M, Guenther H L, Fleisch H, Collin P, Martin T J. J Clin Invest. 1993;91:2004–2011. doi: 10.1172/JCI116422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes D E, Wright K R, Uy H L, Sasaki A, Yoneda T, Roodman G D, Mundy G R, Boyce B F. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 4.van Beek E, Hoekstra M, van der Ruit M, Lowik C, Papapoulos S. J Bone Miner Res. 1994;9:1875–1882. doi: 10.1002/jbmr.5650091206. [DOI] [PubMed] [Google Scholar]
- 5.Rogers M J, Xiong X, Brown R J, Watts D J, Russell R G G, Bayless A V, Ebetino F H. Mol Pharmacol. 1995;47:398–402. [PubMed] [Google Scholar]
- 6.Rogers M J, Chilton K M, Coxon F P, Lawry J, Smith M O, Suri S, Russell R G G. J Bone Miner Res. 1996;11:1482–1491. doi: 10.1002/jbmr.5650111015. [DOI] [PubMed] [Google Scholar]
- 7.Luckman S P, Hughes D E, Coxon F P, Russell R G G, Rogers M J. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A, Rutledge S J, Endo N, Opas E E, Tanaka H, Wesolowski G, Leu C-T, Huang Z, Ramachandaran C, Rodan S B, et al. Proc Natl Acad Sci USA. 1996;93:3068–3073. doi: 10.1073/pnas.93.7.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin D, Cornell S A, Gustafson S K, Needle S J, Ullrich J W, Bilder G E, Perrone M H. J Lipid Res. 1992;33:1657–1663. [PubMed] [Google Scholar]
- 10.Olkkonen V M, Stenmark H. Int Rev Cytol. 1997;176:1–85. doi: 10.1016/s0074-7696(08)61608-3. [DOI] [PubMed] [Google Scholar]
- 11.Glomset J A, Gelb M H, Farnsworth C C. Biochem Soc Trans. 1992;20:479–484. doi: 10.1042/bst0200479. [DOI] [PubMed] [Google Scholar]
- 12.Sato M, Grasser W, Endo N, Atkins R, Simmons H, Thompson D D, Golub E, Rodan G A. J Clin Invest. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wesolowski G, Duong L T, Lakkakorpi P T, Nagy R M, Tezuka K, Tanaka H, Rodan G A, Rodan S B. Exp Cell Res. 1995;219:679–686. doi: 10.1006/excr.1995.1279. [DOI] [PubMed] [Google Scholar]
- 14.Garret I R, Boyce B F, Oreffo R O C, Bonewald L, Poser J, Mundy G R. J Clin Invest. 1990;85:632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kameshita I, Fujisawa H. Anal Biochem. 1989;183:139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- 16.Gotoh Y, Nishida E, Yamashita T, Hoshi M, Kawakami M, Sakai H. Eur J Biochem. 1990;793:661–669. doi: 10.1111/j.1432-1033.1990.tb19384.x. [DOI] [PubMed] [Google Scholar]
- 17.Crick D C, Andres D A, Waechter C J. Biochem Biophys Res Commun. 1997;237:483–487. doi: 10.1006/bbrc.1997.7145. [DOI] [PubMed] [Google Scholar]
- 18.Brown M S, Goldstein J L. Nature (London) 1993;366:14–15. doi: 10.1038/366014a0. [DOI] [PubMed] [Google Scholar]
- 19.Teitelbaum S L, Yousef A-A, Ross F P. J Cell Biochem. 1995;59:1–10. doi: 10.1002/jcb.240590102. [DOI] [PubMed] [Google Scholar]
- 20.Frith J C, Monkkonen J, Blackburn G M, Russell R G G, Rogers M J. J Bone Miner Res. 1997;12:1358–1367. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- 21.Salo J, Lehenkari P, Mulari M, Metsikko K, Vaananen H K. Science. 1997;276:270–273. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- 22.Masarachia P, Weinreb M, Balena R, Rodan G A. Bone. 1996;19:281–290. doi: 10.1016/8756-3282(96)00182-2. [DOI] [PubMed] [Google Scholar]
- 23.Murakami H, Takahashi N, Sasaki T, Udagawa N, Tanaka S, Nakamura I, Zhang D, Barbier A, Suda T. Bone. 1995;17:137–144. doi: 10.1016/s8756-3282(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 24.Rogers M J, Xiong X J, Ji X H, Monkkonen J, Russell R G G, Williamson M P, Ebetino F H, Watts D J. Pharm Res. 1997;14:625–630. doi: 10.1023/a:1012157212793. [DOI] [PubMed] [Google Scholar]