Abstract
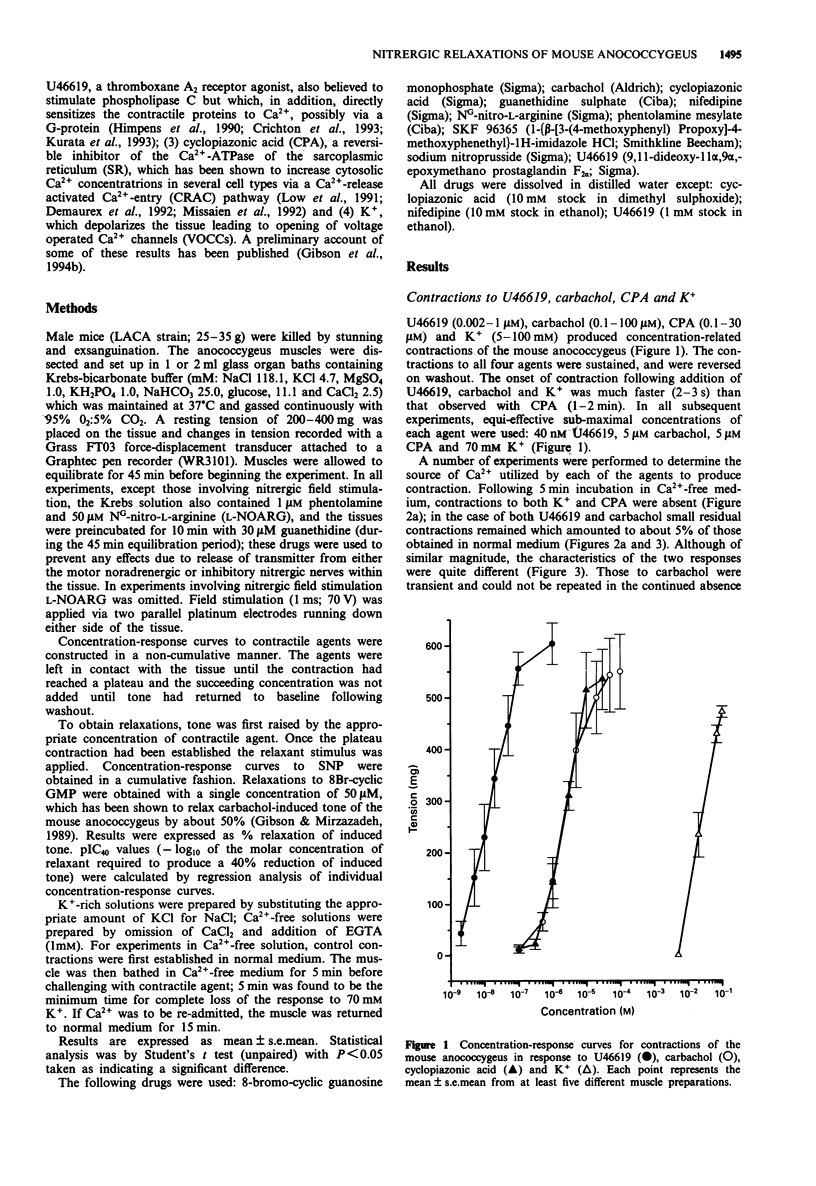
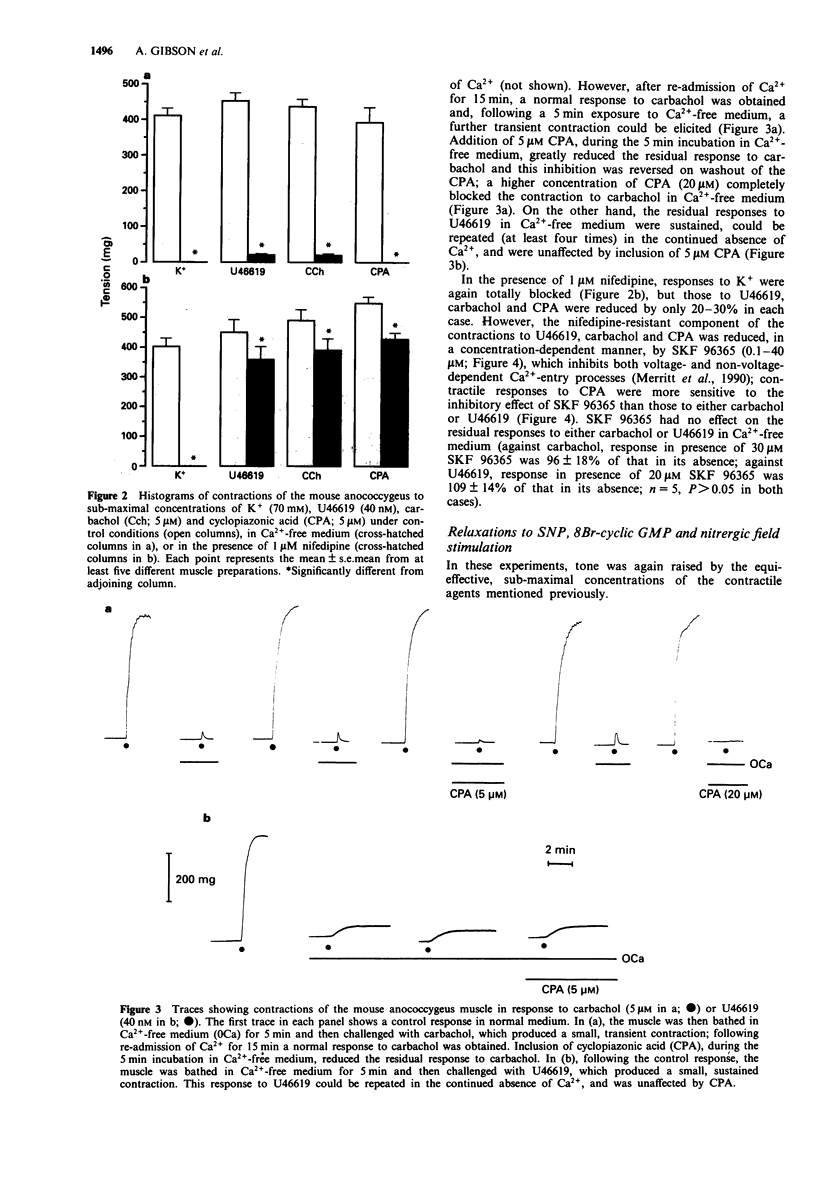
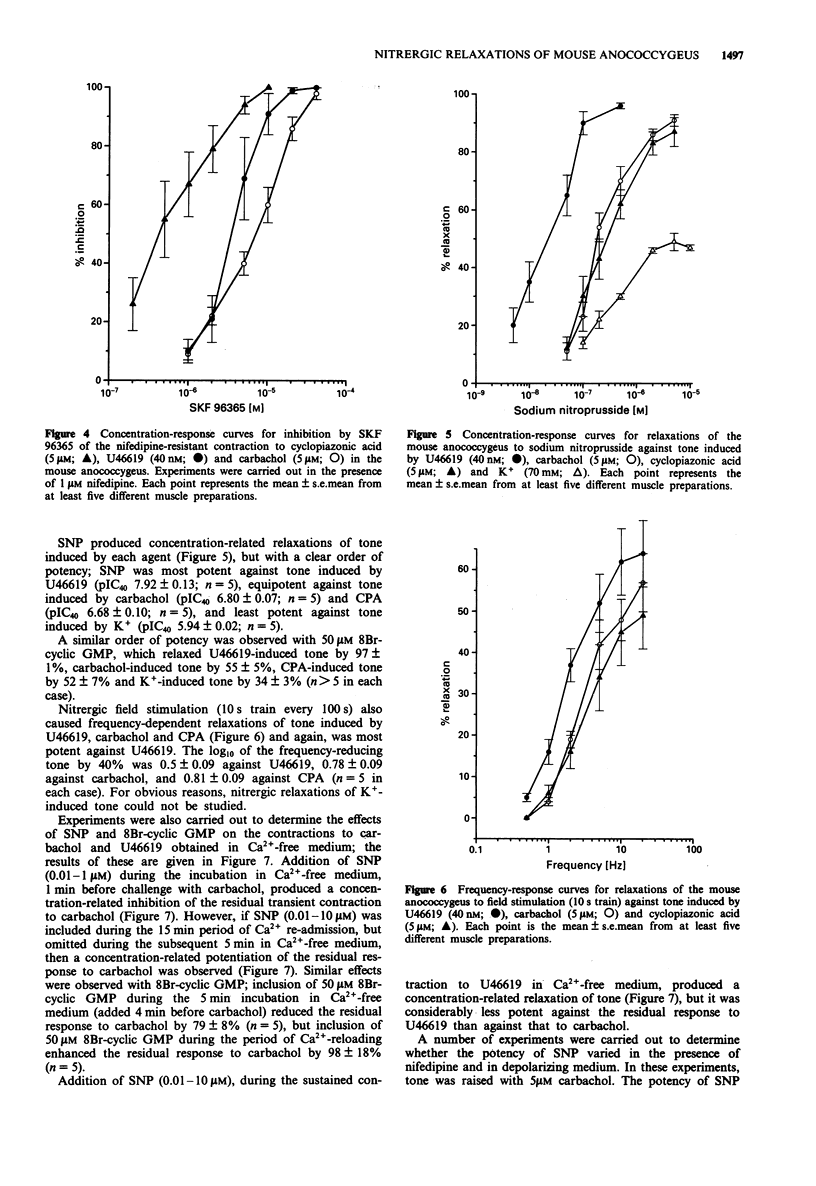
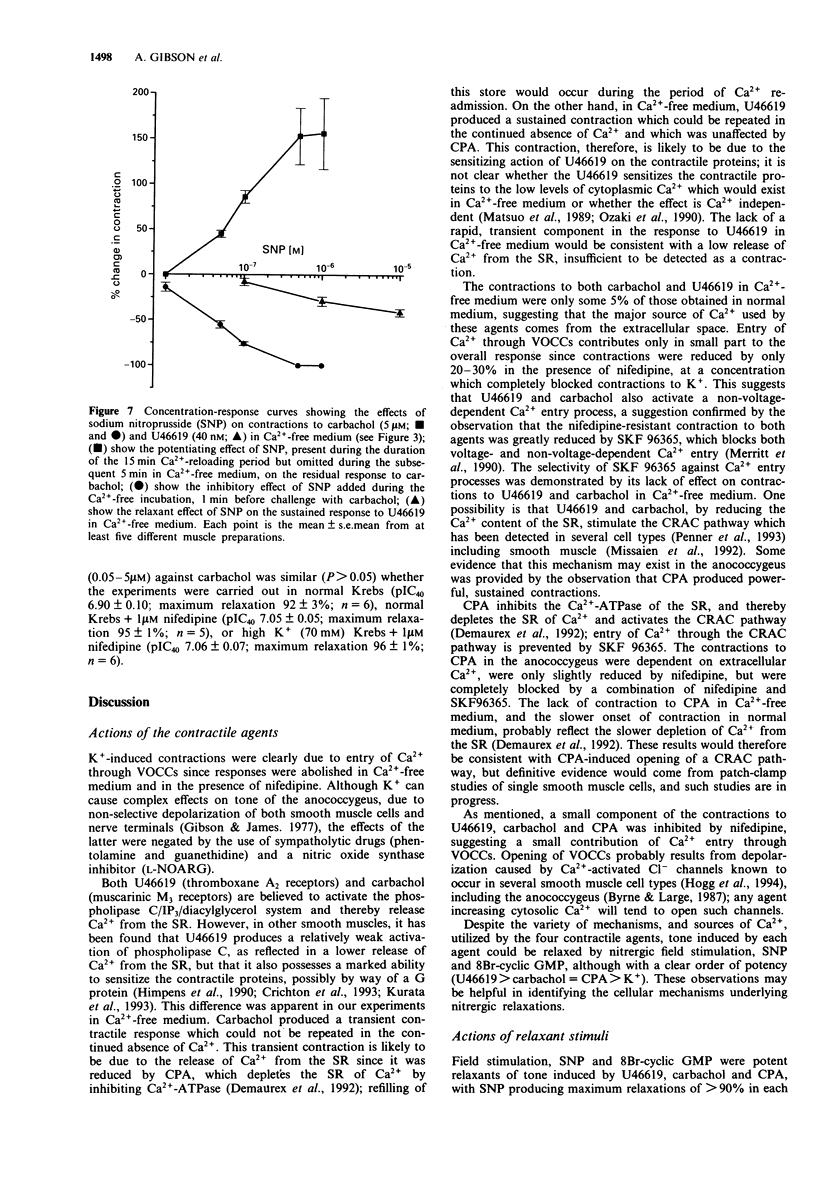
1. U46619 (thromboxane A2 receptors; 0.002-1 microM), carbachol (muscarinic M3 receptors; 0.1-100 microM), cyclopiazonic acid (CPA; Ca(2+)-ATPase inhibitor; 0.1-30 microM) and K+ (5-100 mM) produced concentration-dependent contractions of the mouse isolated anococcygeus muscle. Equi-effective, submaximal concentrations of each agent were used in further experiments (40 nM U46619; 5 microM carbachol; 5 microM CPA; 70 mM K+). 2. Nifedipine (1 microM) totally abolished contractile responses to K+; those to U46619, carbachol and CPA were reduced by only 20-30% in the presence of nifedipine, but were greatly reduced (> 90%) by a combination of nifedipine and SKF 96365 (0.1-40 microM). 3. In Ca(2+)-free medium, contractions to K+ and CPA were abolished. Small residual responses remained to both carbachol and U46619; those to carbachol were transient, could not be repeated in the continued absence of Ca2+ and were prevented by pre-incubation with CPA, but unaffected by SKF 96365; those to U46619 were sustained, could be repeated in the absence of Ca2+, and were resistant to CPA and SKF 96365. 4. Tone induced by all four agents could be relaxed by sodium nitroprusside (SNP), but with a clear order of potency. SNP (pIC40) was most effective against U46619 (7.92), less so against carbachol (6.80) and CPA (6.68), and least potent against K+ (5.94). A similar order of potency was observed with 8Br-cyclic GMP (50 microM) and nitrergic field stimulation (1-20 Hz). 5. The relaxant potency of SNP was similar in normal Krebs solution and in high K+ (70 mM) Krebs containing 1 microM nifedipine.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar R. A., Abdel-Latif A. A. The effect of M & B 22948 on carbachol-induced inositol trisphosphate accumulation and contraction in iris sphincter smooth muscle. Eur J Pharmacol. 1991 Apr 25;206(4):291–295. doi: 10.1016/0922-4106(91)90112-u. [DOI] [PubMed] [Google Scholar]
- Bourreau J. P., Kwan C. Y., Daniel E. E. Distinct pathways to refill ACh-sensitive internal Ca2+ stores in canine airway smooth muscle. Am J Physiol. 1993 Jul;265(1 Pt 1):C28–C35. doi: 10.1152/ajpcell.1993.265.1.C28. [DOI] [PubMed] [Google Scholar]
- Brave S. R., Tucker J. F., Gibson A., Bishop A. E., Riveros-Moreno V., Moncada S., Polak J. M. Localisation of nitric oxide synthase within non-adrenergic, non-cholinergic nerves in the mouse anococcygeus. Neurosci Lett. 1993 Oct 14;161(1):93–96. doi: 10.1016/0304-3940(93)90148-e. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Membrane mechanism associated with muscarinic receptor activation in single cells freshly dispersed from the rat anococcygeus muscle. Br J Pharmacol. 1987 Oct;92(2):371–379. doi: 10.1111/j.1476-5381.1987.tb11333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilvers E. R., Giembycz M. A., Challiss R. A., Barnes B. J., Nahorski S. R. Lack of effect of zaprinast on methacholine-induced contraction and inositol 1,4,5-trisphosphate accumulation in bovine tracheal smooth muscle. Br J Pharmacol. 1991 May;103(1):1119–1125. doi: 10.1111/j.1476-5381.1991.tb12310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell T. L., Pryzwansky K. B., Wyatt T. A., Lincoln T. M. Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol Pharmacol. 1991 Dec;40(6):923–931. [PubMed] [Google Scholar]
- Crichton C. A., Templeton A. G., McGrath J. C., Smith G. L. Thromboxane A2 analogue, U-46619, potentiates calcium-activated force in human umbilical artery. Am J Physiol. 1993 Jun;264(6 Pt 2):H1878–H1883. doi: 10.1152/ajpheart.1993.264.6.H1878. [DOI] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Fasolato C., Innocenti B., Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994 Mar;15(3):77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- García-Pascual A., Triguero D. Relaxation mechanisms induced by stimulation of nerves and by nitric oxide in sheep urethral muscle. J Physiol. 1994 Apr 15;476(2):333–347. doi: 10.1113/jphysiol.1994.sp020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Brave S. R., McFadzean I., Mirzazadeh S., Tucker J. F., Wayman C. Nitrergic stimulation does not inhibit carbachol-induced inositol phosphate generation in the rat anococcygeus. Neurosci Lett. 1994 Aug 29;178(1):35–38. doi: 10.1016/0304-3940(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Gibson A., James T. A. The nature of potassium chloride-induced relaxations of the rat anococcygeus muscle. Br J Pharmacol. 1977 May;60(1):141–145. doi: 10.1111/j.1476-5381.1977.tb16758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S., Hobbs A. J., Moore P. K. L-NG-monomethyl arginine and L-NG-nitro arginine inhibit non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br J Pharmacol. 1990 Mar;99(3):602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S. N-methylhydroxylamine inhibits and M&B 22948 potentiates relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs. Br J Pharmacol. 1989 Mar;96(3):637–644. doi: 10.1111/j.1476-5381.1989.tb11863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpens B., Kitazawa T., Somlyo A. P. Agonist-dependent modulation of Ca2+ sensitivity in rabbit pulmonary artery smooth muscle. Pflugers Arch. 1990 Sep;417(1):21–28. doi: 10.1007/BF00370764. [DOI] [PubMed] [Google Scholar]
- Hogg R. C., Wang Q., Large W. A. Effects of Cl channel blockers on Ca-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1994 Apr;111(4):1333–1341. doi: 10.1111/j.1476-5381.1994.tb14891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata R., Takayanagi I., Hisayama T. Eicosanoid-induced Ca2+ release and sustained contraction in Ca(2+)-free media are mediated by different signal transduction pathways in rat aorta. Br J Pharmacol. 1993 Oct;110(2):875–881. doi: 10.1111/j.1476-5381.1993.tb13894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1989 Dec;16(12):933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L. Intracellular cyclic GMP receptor proteins. FASEB J. 1993 Feb 1;7(2):328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M. Cyclic GMP and mechanisms of vasodilation. Pharmacol Ther. 1989;41(3):479–502. doi: 10.1016/0163-7258(89)90127-7. [DOI] [PubMed] [Google Scholar]
- Low A. M., Gaspar V., Kwan C. Y., Darby P. J., Bourreau J. P., Daniel E. E. Thapsigargin inhibits repletion of phenylephrine-sensitive intracellular Ca++ pool in vascular smooth muscles. J Pharmacol Exp Ther. 1991 Sep;258(3):1105–1113. [PubMed] [Google Scholar]
- Matsuo K., Gokita T., Karibe H., Uchida M. K. Ca2+-independent contraction of uterine smooth muscle. Biochem Biophys Res Commun. 1989 Dec 15;165(2):722–727. doi: 10.1016/s0006-291x(89)80026-9. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Armstrong W. P., Benham C. D., Hallam T. J., Jacob R., Jaxa-Chamiec A., Leigh B. K., McCarthy S. A., Moores K. E., Rink T. J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990 Oct 15;271(2):515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzazadeh S., Hobbs A. J., Tucker J. F., Gibson A. Cyclic nucleotide content of the rat anococcygeus during relaxations induced by drugs or by non-adrenergic, non-cholinergic field stimulation. J Pharm Pharmacol. 1991 Apr;43(4):247–251. doi: 10.1111/j.2042-7158.1991.tb06677.x. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Himpens B., Casteels R. Calcium ion homeostasis in smooth muscle. Pharmacol Ther. 1992 Nov;56(2):191–231. doi: 10.1016/0163-7258(92)90017-t. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Sheng H., Förstermann U., Murad F. Characterization of nitric oxide synthases in non-adrenergic non-cholinergic nerve containing tissue from the rat anococcygeus muscle. Br J Pharmacol. 1991 Oct;104(2):289–291. doi: 10.1111/j.1476-5381.1991.tb12422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989 Sep 15;163(2):929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Ohyama T., Sato K., Karaki H. Ca2(+)-dependent and independent mechanisms of sustained contraction in vascular smooth muscle of rat aorta. Jpn J Pharmacol. 1990 Mar;52(3):509–512. doi: 10.1254/jjp.52.509. [DOI] [PubMed] [Google Scholar]
- Penner R., Fasolato C., Hoth M. Calcium influx and its control by calcium release. Curr Opin Neurobiol. 1993 Jun;3(3):368–374. doi: 10.1016/0959-4388(93)90130-q. [DOI] [PubMed] [Google Scholar]
- Pfitzer G., Hofmann F., DiSalvo J., Rüegg J. C. cGMP and cAMP inhibit tension development in skinned coronary arteries. Pflugers Arch. 1984 Jul;401(3):277–280. doi: 10.1007/BF00582596. [DOI] [PubMed] [Google Scholar]
- Ramagopal M. V., Leighton H. J. Effects of NG-monomethyl-L-arginine on field stimulation-induced decreases in cytosolic Ca2+ levels and relaxation in the rat anococcygeus muscle. Eur J Pharmacol. 1989 Dec 19;174(2-3):297–299. doi: 10.1016/0014-2999(89)90325-7. [DOI] [PubMed] [Google Scholar]
- Rand M. J. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin Exp Pharmacol Physiol. 1992 Mar;19(3):147–169. doi: 10.1111/j.1440-1681.1992.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Thornbury K. D., Ward S. M., Dalziel H. H., Carl A., Westfall D. P., Sanders K. M. Nitric oxide and nitrosocysteine mimic nonadrenergic, noncholinergic hyperpolarization in canine proximal colon. Am J Physiol. 1991 Sep;261(3 Pt 1):G553–G557. doi: 10.1152/ajpgi.1991.261.3.G553. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]


