Abstract
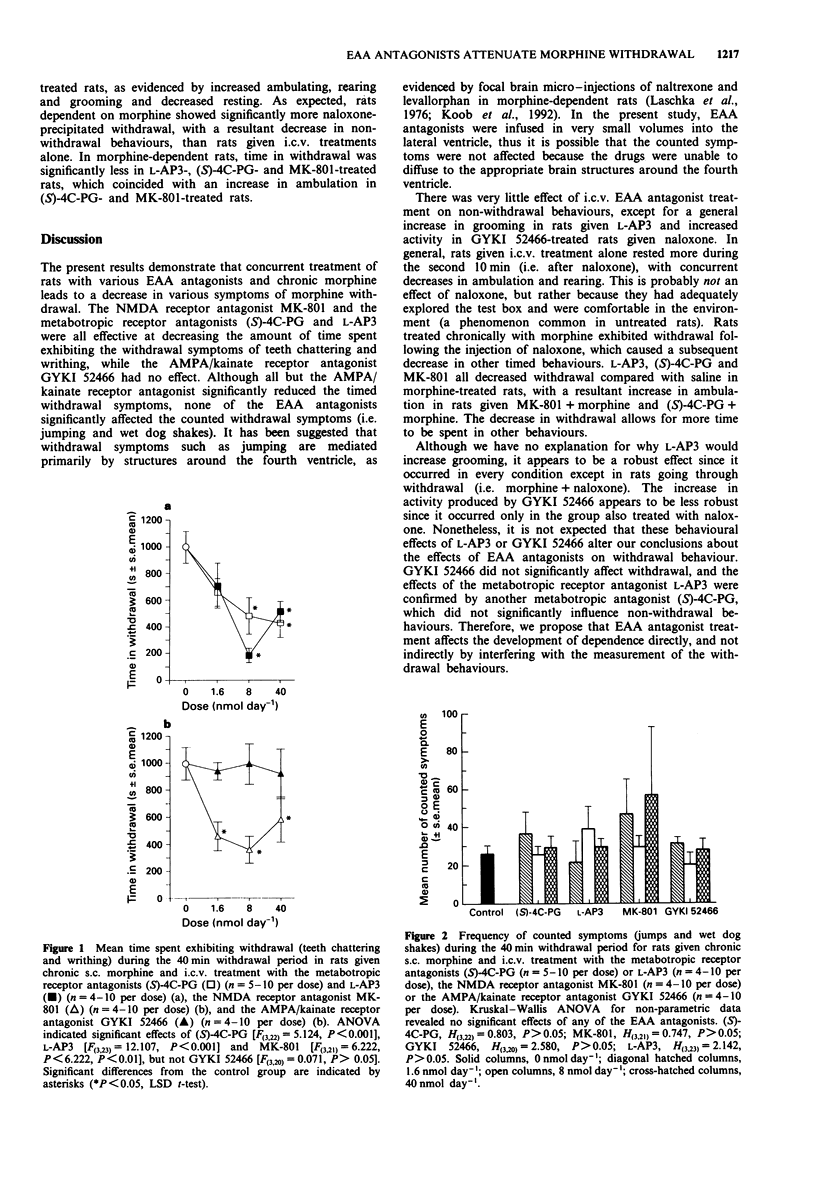
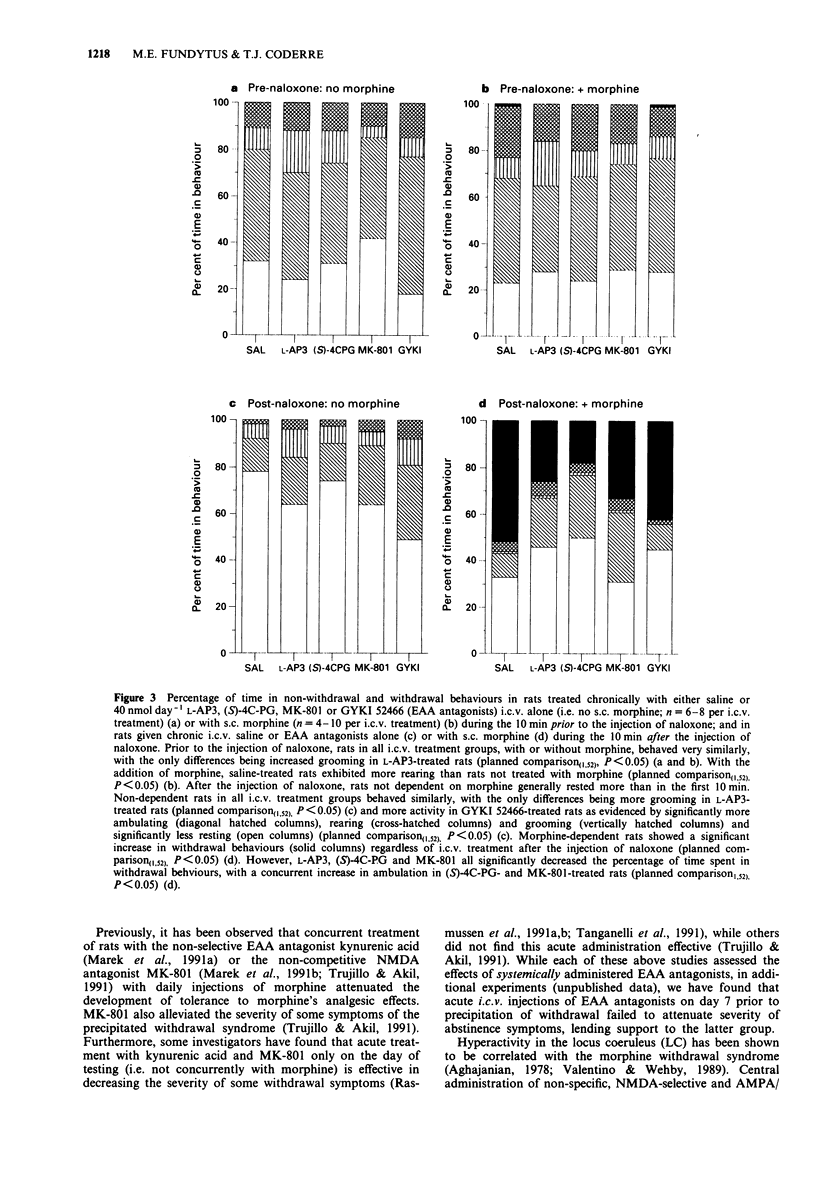
1. The contribution of various excitatory amino acid (EAA) receptors (NMDA, AMPA/kainate and metabotropic) in the brain to the development of morphine dependence was examined. This was performed by measuring the severity of the precipitated withdrawal syndrome following chronic subcutaneous (s.c.) morphine and intracerebroventricular (i.c.v.) EAA antagonist treatment. 2. Continuous subcutaneous (s.c.) treatment with morphine sulphate (36.65 mumol day-1) produced an intense and reliable naloxone-precipitated withdrawal syndrome. 3. Chronic i.c.v. treatment with antagonists selective for metabotropic and NMDA receptors, but not AMPA/kainate receptors, significantly attenuated abstinence symptoms. Conversely, EAA antagonists had very little effect on non-withdrawal behaviours. 4. These results suggest that, as well as changes elicited by activation of NMDA receptors, metabotropic receptors and intracellular changes in the phosphatidylinositol (PI) second-messenger system or the cyclic adenosine 3',5'-monophosphate (cAMP) second messenger system, to which EAA metabotropic receptors are linked, may be involved in the development of opioid dependence with chronic morphine treatment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978 Nov 9;276(5684):186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Akaoka H., Aston-Jones G. Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J Neurosci. 1991 Dec;11(12):3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini A., Meldolesi J. Muscarinic and quisqualate receptor-induced phosphoinositide hydrolysis in primary cultures of striatal and hippocampal neurons. Evidence for differential mechanisms of activation. J Neurochem. 1989 Sep;53(3):825–833. doi: 10.1111/j.1471-4159.1989.tb11779.x. [DOI] [PubMed] [Google Scholar]
- Birse E. F., Eaton S. A., Jane D. E., Jones P. L., Porter R. H., Pook P. C., Sunter D. C., Udvarhelyi P. M., Wharton B., Roberts P. J. Phenylglycine derivatives as new pharmacological tools for investigating the role of metabotropic glutamate receptors in the central nervous system. Neuroscience. 1993 Feb;52(3):481–488. doi: 10.1016/0306-4522(93)90400-a. [DOI] [PubMed] [Google Scholar]
- Bläsig J., Herz A., Reinhold K., Zieglgänsberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973 Oct 23;33(1):19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- Collier H. O. Cellular site of opiate dependence. Nature. 1980 Feb 14;283(5748):625–629. doi: 10.1038/283625a0. [DOI] [PubMed] [Google Scholar]
- Collier H. O. Physiological basis of opiate dependence. Drug Alcohol Depend. 1983 Feb;11(1):15–21. doi: 10.1016/0376-8716(83)90089-3. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Monaghan D. T., Ganong A. H. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annu Rev Neurosci. 1988;11:61–80. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- Dixon W., Ting Y. W., Chang A. P. The effects of morphine on norepinephrine-stimulated phosphatidylinositol response in rat cerebral cortex. Prog Clin Biol Res. 1990;328:279–282. [PubMed] [Google Scholar]
- Donevan S. D., Rogawski M. A. GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron. 1993 Jan;10(1):51–59. doi: 10.1016/0896-6273(93)90241-i. [DOI] [PubMed] [Google Scholar]
- Eaton S. A., Jane D. E., Jones P. L., Porter R. H., Pook P. C., Sunter D. C., Udvarhelyi P. M., Roberts P. J., Salt T. E., Watkins J. C. Competitive antagonism at metabotropic glutamate receptors by (S)-4-carboxyphenylglycine and (RS)-alpha-methyl-4-carboxyphenylglycine. Eur J Pharmacol. 1993 Jan 15;244(2):195–197. doi: 10.1016/0922-4106(93)90028-8. [DOI] [PubMed] [Google Scholar]
- Jonas P., Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol. 1992 Sep;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskin N. I., Krishtal O. A., Tsyndrenko AYa Excitatory amino acid receptors in hippocampal neurons: kainate fails to desensitize them. Neurosci Lett. 1986 Jan 30;63(3):225–230. doi: 10.1016/0304-3940(86)90360-5. [DOI] [PubMed] [Google Scholar]
- Koob G. F., Maldonado R., Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992 May;15(5):186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Laschka E., Teschemacher H., Mehraein P., Herz A. Sites of action of morphine involved in the development of physical dependence in rats. II. Morphine withdrawal precipitated by application of morphine antagonists into restricted parts of the ventricular system and by microinjection into various brain areas. Psychopharmacologia. 1976 Mar 16;46(2):141–147. doi: 10.1007/BF00421383. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Manzoni O. J., Finiels-Marlier F., Sassetti I., Blockaert J., le Peuch C., Sladeczek F. A. The glutamate receptor of the Qp-type activates protein kinase C and is regulated by protein kinase C. Neurosci Lett. 1990 Feb 5;109(1-2):146–151. doi: 10.1016/0304-3940(90)90553-l. [DOI] [PubMed] [Google Scholar]
- Marek P., Ben-Eliyahu S., Gold M., Liebeskind J. C. Excitatory amino acid antagonists (kynurenic acid and MK-801) attenuate the development of morphine tolerance in the rat. Brain Res. 1991 Apr 26;547(1):77–81. doi: 10.1016/0006-8993(91)90576-h. [DOI] [PubMed] [Google Scholar]
- Marek P., Ben-Eliyahu S., Vaccarino A. L., Liebeskind J. C. Delayed application of MK-801 attenuates development of morphine tolerance in rats. Brain Res. 1991 Aug 30;558(1):163–165. doi: 10.1016/0006-8993(91)90736-f. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Miller R. J. The revenge of the kainate receptor. Trends Neurosci. 1991 Nov;14(11):477–479. doi: 10.1016/0166-2236(91)90054-x. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Murphy S. N., Miller R. J. Regulation of Ca++ influx into striatal neurons by kainic acid. J Pharmacol Exp Ther. 1989 Apr;249(1):184–193. [PubMed] [Google Scholar]
- Raffa R. B., Martinez R. P. Morphine antinociception is mediated through a LiCl-sensitive, IP3-restorable pathway. Eur J Pharmacol. 1992 May 14;215(2-3):357–358. doi: 10.1016/0014-2999(92)90060-h. [DOI] [PubMed] [Google Scholar]
- Rasmussen K., Fuller R. W., Stockton M. E., Perry K. W., Swinford R. M., Ornstein P. L. NMDA receptor antagonists suppress behaviors but not norepinephrine turnover or locus coeruleus unit activity induced by opiate withdrawal. Eur J Pharmacol. 1991 May 2;197(1):9–16. doi: 10.1016/0014-2999(91)90358-w. [DOI] [PubMed] [Google Scholar]
- Rasmussen K., Krystal J. H., Aghajanian G. K. Excitatory amino acids and morphine withdrawal: differential effects of central and peripheral kynurenic acid administration. Psychopharmacology (Berl) 1991;105(4):508–512. doi: 10.1007/BF02244371. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D., Conn P. J. Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci. 1993 Jan;14(1):13–20. doi: 10.1016/0165-6147(93)90107-u. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D., Johnson B. G., Smith E. C., McQuaid L. A. Stereoselectivity and mode of inhibition of phosphoinositide-coupled excitatory amino acid receptors by 2-amino-3-phosphonopropionic acid. Mol Pharmacol. 1990 Aug;38(2):222–228. [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladeczek F., Pin J. P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985 Oct 24;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Sommer B., Seeburg P. H. Glutamate receptor channels: novel properties and new clones. Trends Pharmacol Sci. 1992 Jul;13(7):291–296. doi: 10.1016/0165-6147(92)90088-n. [DOI] [PubMed] [Google Scholar]
- Steppuhn K. G., Turski L. Diazepam dependence prevented by glutamate antagonists. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6889–6893. doi: 10.1073/pnas.90.14.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Tanganelli S., Antonelli T., Morari M., Bianchi C., Beani L. Glutamate antagonists prevent morphine withdrawal in mice and guinea pigs. Neurosci Lett. 1991 Jan 28;122(2):270–272. doi: 10.1016/0304-3940(91)90875-t. [DOI] [PubMed] [Google Scholar]
- Trujillo K. A., Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991 Jan 4;251(4989):85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- Valentino R. J., Wehby R. G. Locus ceruleus discharge characteristics of morphine-dependent rats: effects of naltrexone. Brain Res. 1989 May 29;488(1-2):126–134. doi: 10.1016/0006-8993(89)90701-4. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]


