Abstract
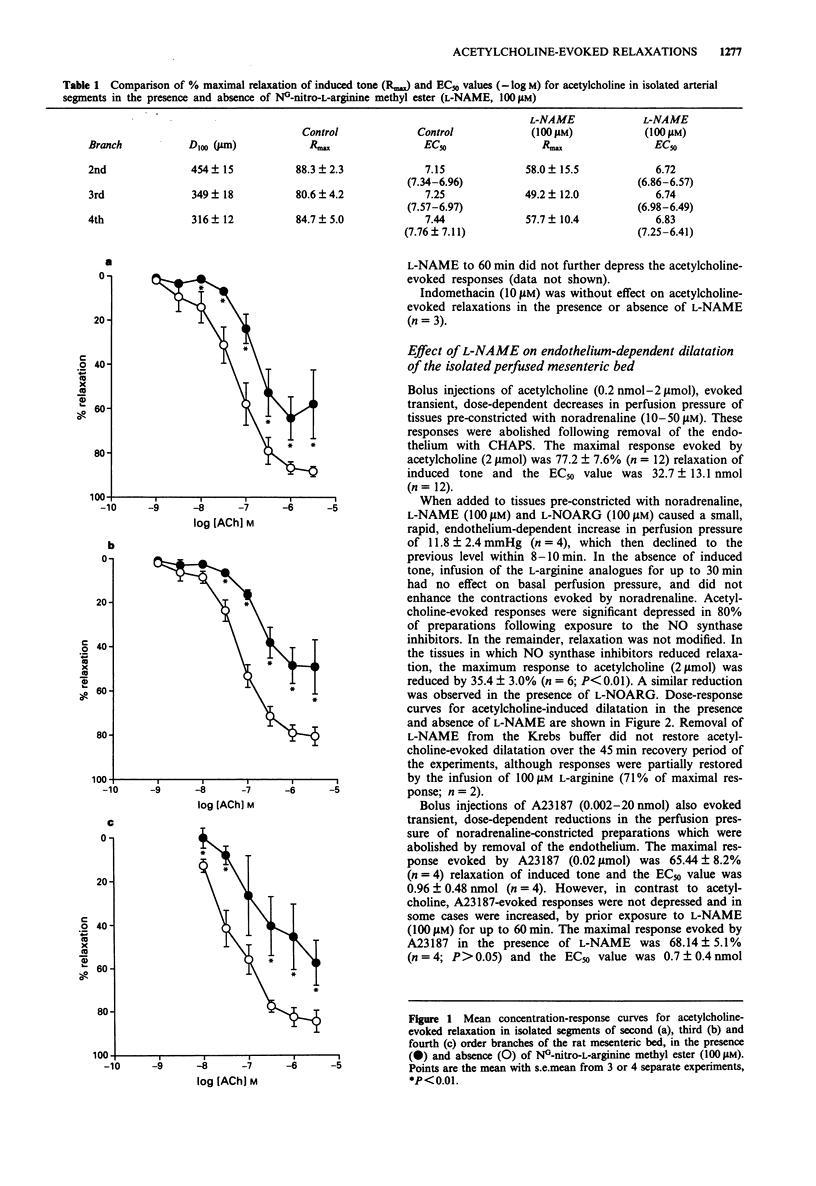
1. The relative contribution of nitric oxide (NO) to acetylcholine-induced smooth muscle relaxation was investigated in the rat perfused mesenteric vasculature and in isolated segments of second, third and fourth order arterial branches. 2. The EC50 values and maximal relaxation to acetylcholine were not significantly different in the sequential arterial branches, being approximately 0.05 microM and 85%, respectively. 3. The NO synthase inhibitor L-NG-nitro-L-arginine methyl ester (L-NAME; 100 microM) reduced acetylcholine-evoked endothelium-dependent dilatation and relaxation in the perfused mesenteric bed and in isolated arterial segments. The maximum response to acetylcholine in both preparations was reduced by between 35% to 40% while the EC50 values were increased by 5-6 fold. L-NAME had no effect on basal smooth muscle tone in either case. 4. In contrast, endothelium-dependent dilatation of the perfused mesenteric bed evoked by A23187 (0.002-20 nmol), was unaffected by exposure to L-NAME. The EC50 values and maximal responses elicited by A23187 (20 nmol) before and after exposure to L-NAME were 0.96 +/- 0.5 nmol and 67.0 +/- 7.0% (n = 4), and 0.7 +/- 0.4 nmol and 70.0 +/- 5.0% (n = 4; P > 0.01), respectively. 5. Perfusion of the isolated mesenteric bed with raised K(+)-Krebs buffer (25 mM) had no effect on basal tone, but reduced the amplitude of both acetylcholine- and A23187-evoked dilatation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

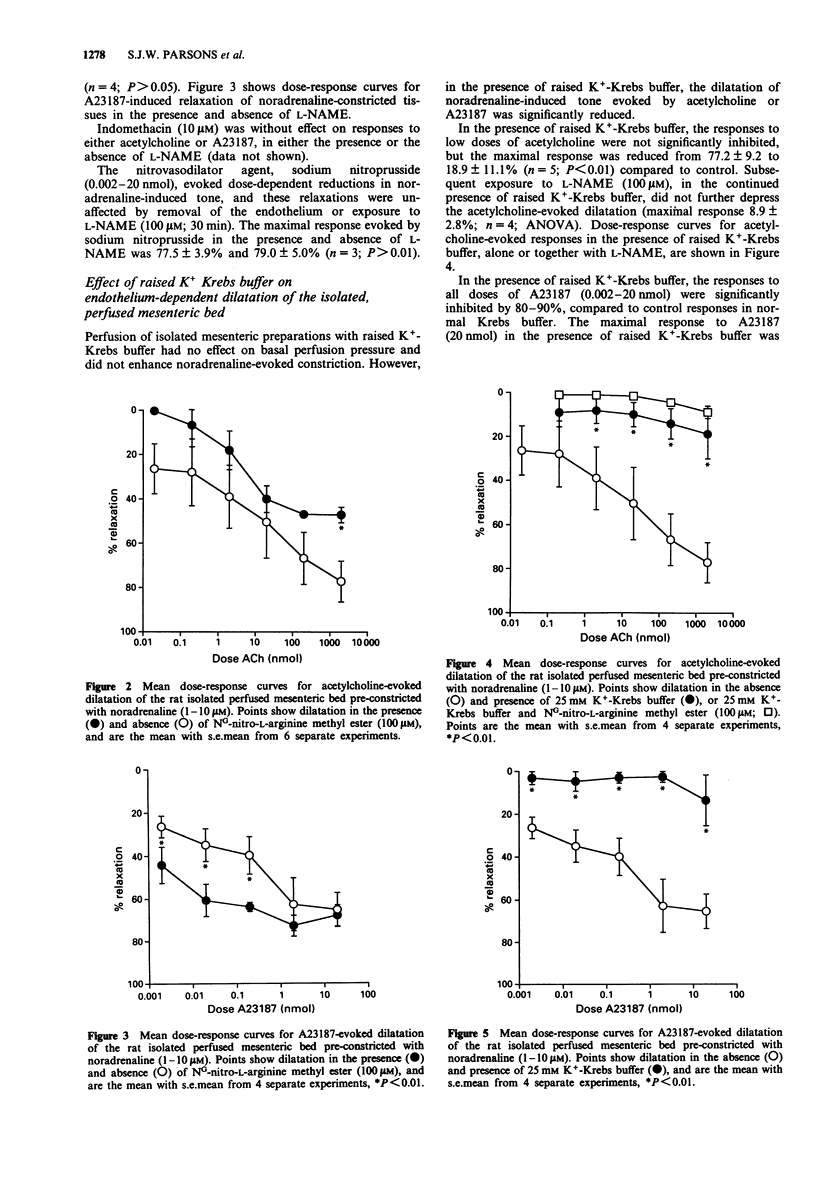


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeagbo A. S., Triggle C. R. Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J Cardiovasc Pharmacol. 1993 Mar;21(3):423–429. [PubMed] [Google Scholar]
- Bennett M. A., Watt P. A., Thurston H. Endothelium-dependent modulation of resistance vessel contraction: studies with NG-nitro-L-arginine methyl ester and NG-nitro-L-arginine. Br J Pharmacol. 1992 Oct;107(2):616–621. doi: 10.1111/j.1476-5381.1992.tb12792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand N., Mahoney T. P., Jr, Diamantis W., Sofia R. D. Pharmacological modulation of bradykinin-, acetylcholine- and calcium ionophore A23187-induced relaxation of rabbit pulmonary arterial segments. Eur J Pharmacol. 1987 Jun 4;137(2-3):173–177. doi: 10.1016/0014-2999(87)90219-6. [DOI] [PubMed] [Google Scholar]
- Chen G., Suzuki H., Weston A. H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988 Dec;95(4):1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Henderson A. H., Lang D., Lewis M. J. Endothelium-derived relaxing factor and nitroprusside compared in noradrenaline- and K+-contracted rabbit and rat aortae. J Physiol. 1988 Jun;400:395–404. doi: 10.1113/jphysiol.1988.sp017127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland C. J., McPherson G. A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992 Feb;105(2):429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R. L., Eraslan A., Saito Y. Differences in EDNO contribution to arteriolar diameters at rest and during functional dilation in striated muscle. Am J Physiol. 1993 Jul;265(1 Pt 2):H146–H151. doi: 10.1152/ajpheart.1993.265.1.H146. [DOI] [PubMed] [Google Scholar]
- Jackson W. F., König A., Dambacher T., Busse R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol. 1993 Jan;264(1 Pt 2):H238–H243. doi: 10.1152/ajpheart.1993.264.1.H238. [DOI] [PubMed] [Google Scholar]
- Khan M. T., Jothianandan D., Matsunaga K., Furchgott R. F. Vasodilation induced by acetylcholine and by glyceryl trinitrate in rat aortic and mesenteric vasculature. J Vasc Res. 1992 Jan-Feb;29(1):20–28. doi: 10.1159/000158927. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nagao T., Illiano S., Vanhoutte P. M. Heterogeneous distribution of endothelium-dependent relaxations resistant to NG-nitro-L-arginine in rats. Am J Physiol. 1992 Oct;263(4 Pt 2):H1090–H1094. doi: 10.1152/ajpheart.1992.263.4.H1090. [DOI] [PubMed] [Google Scholar]
- Nagao T., Vanhoutte P. M. Hyperpolarization as a mechanism for endothelium-dependent relaxations in the porcine coronary artery. J Physiol. 1992 Jan;445:355–367. doi: 10.1113/jphysiol.1992.sp018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Parsons A. A., Schilling L., Wahl M. Analysis of acetylcholine-induced relaxation of rabbit isolated middle cerebral artery: effects of inhibitors of nitric oxide synthesis, Na,K-ATPase, and ATP-sensitive K channels. J Cereb Blood Flow Metab. 1991 Jul;11(4):700–704. doi: 10.1038/jcbfm.1991.123. [DOI] [PubMed] [Google Scholar]
- Plane F., Garland C. J. Differential effects of acetylcholine, nitric oxide and levcromakalim on smooth muscle membrane potential and tone in the rabbit basilar artery. Br J Pharmacol. 1993 Oct;110(2):651–656. doi: 10.1111/j.1476-5381.1993.tb13861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T., Joyner W. L. Differential role of endothelial function on vasodilator responses in series-arranged arterioles. Microvasc Res. 1992 Jul;44(1):61–72. doi: 10.1016/0026-2862(92)90102-u. [DOI] [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]


