Abstract
1 The pharmacological properties of the novel diarylacetamide κ-opioid receptor agonist, EMD 61753, have been compared with those of ICI 197067 (a centrally-acting κ agonist) and ICI 204448 (a peripherally-selective κ agonist).
2 EMD 61753 binds with high affinity (IC50 5.6 nM) and selectivity (κ:μ:δ:σ binding ratio 1:536:125:>1,786) to κ-opioid receptors and is a full and potent (IC50 54.5 nM) agonist in an in vitro assay for κ-opioid receptors (rabbit vas deferens preparation).
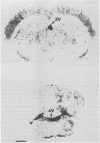
3 Systemically-applied [14C]-EMD 61753 is found in high concentrations in the lungs, liver, adrenal glands and kidneys. Considerably less radioactivity is detected in the whole brain, and this radioactivity is concentrated in the region of the cerebral ventricles in the choroid plexuses. EMD 61753 penetrates only poorly into the CNS.
4 EMD 61753 was weakly effective in pharmacological tests of central activity. This compound reversed haloperidolol-induced DOPA accumulation in the nucleus accumbens of the rat only at a dose of 30 mg kg-1, s.c., (doses of 0.1, 1.0 and 10 mg kg-1, s.c., and 1.0, 10 and 100 mg kg-1, p.o., were inactive). Hexobarbitone-induced sleeping in mice was prolonged by EMD 61753 at threshold doses of 10 mg kg-1, s.c., and 100 mg kg-1, p.o., whereas the motor performance of rats in the rotarod test was impaired by EMD 61753 with an ID50 value of 453 mg kg-1, s.c.
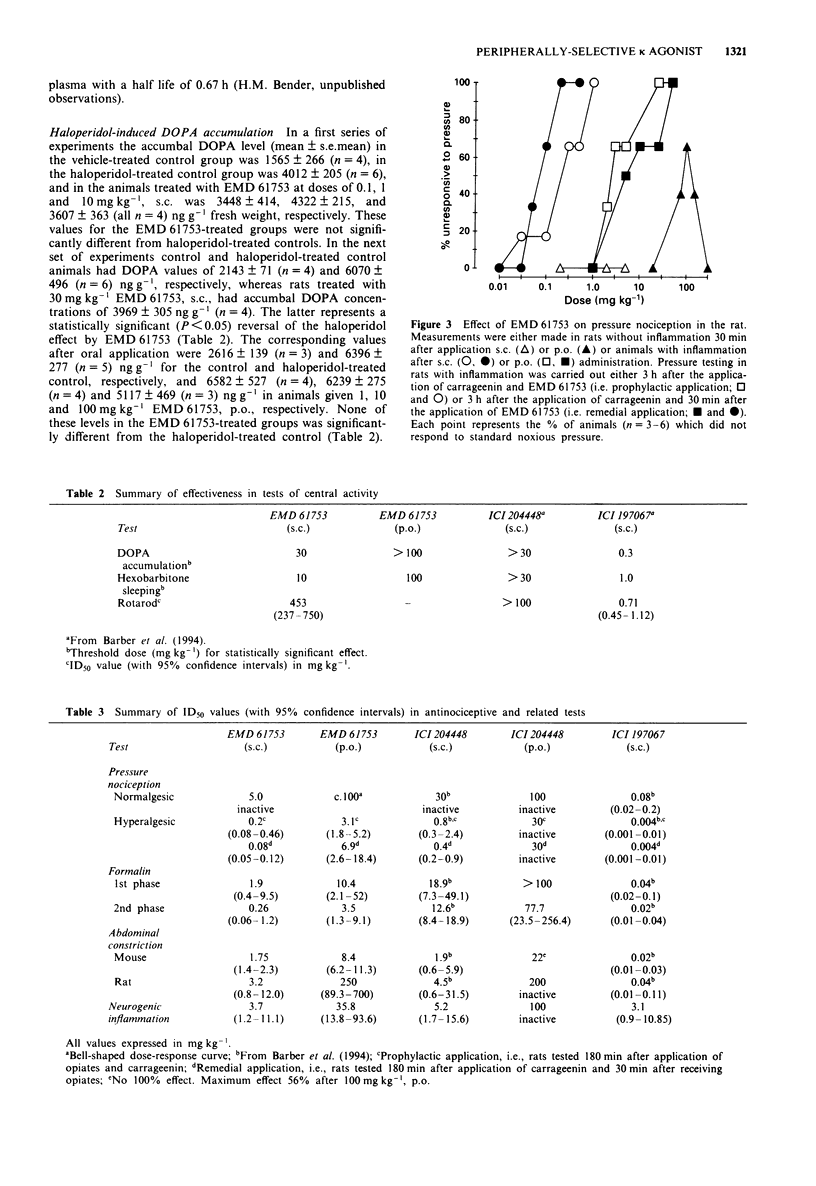
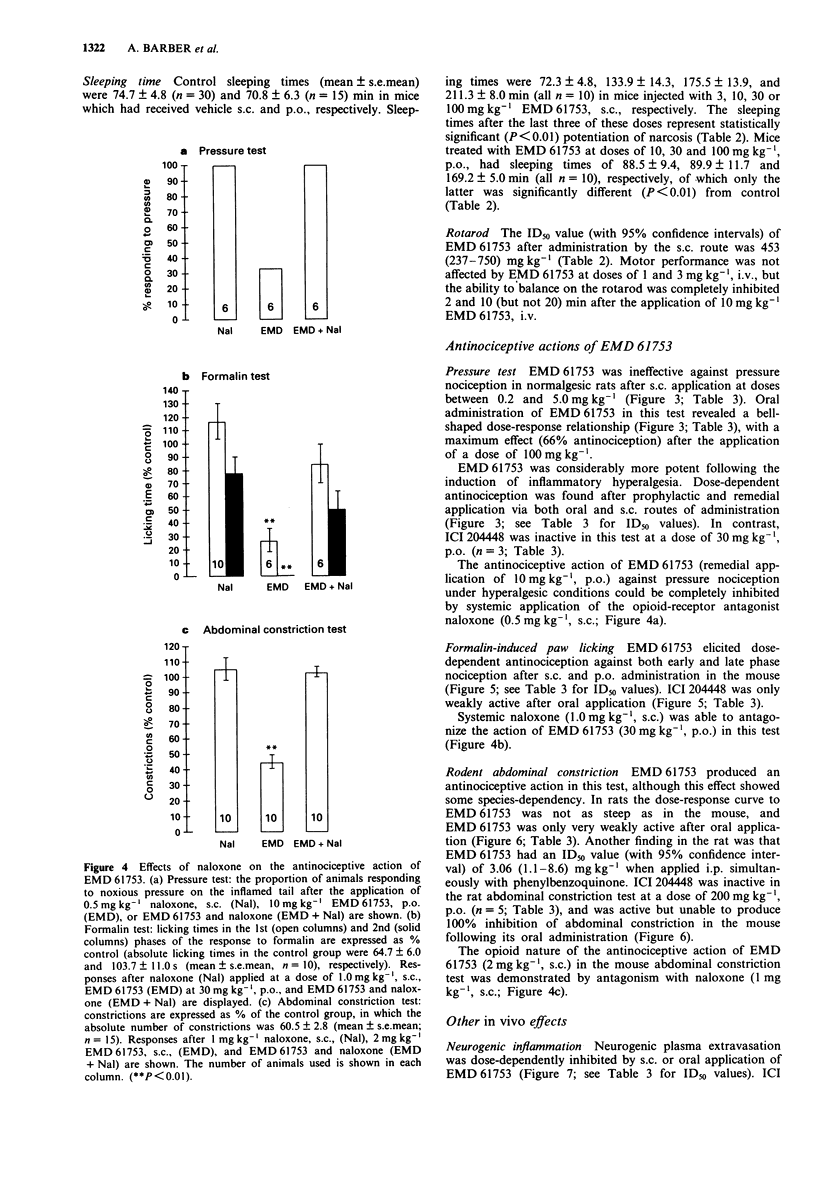
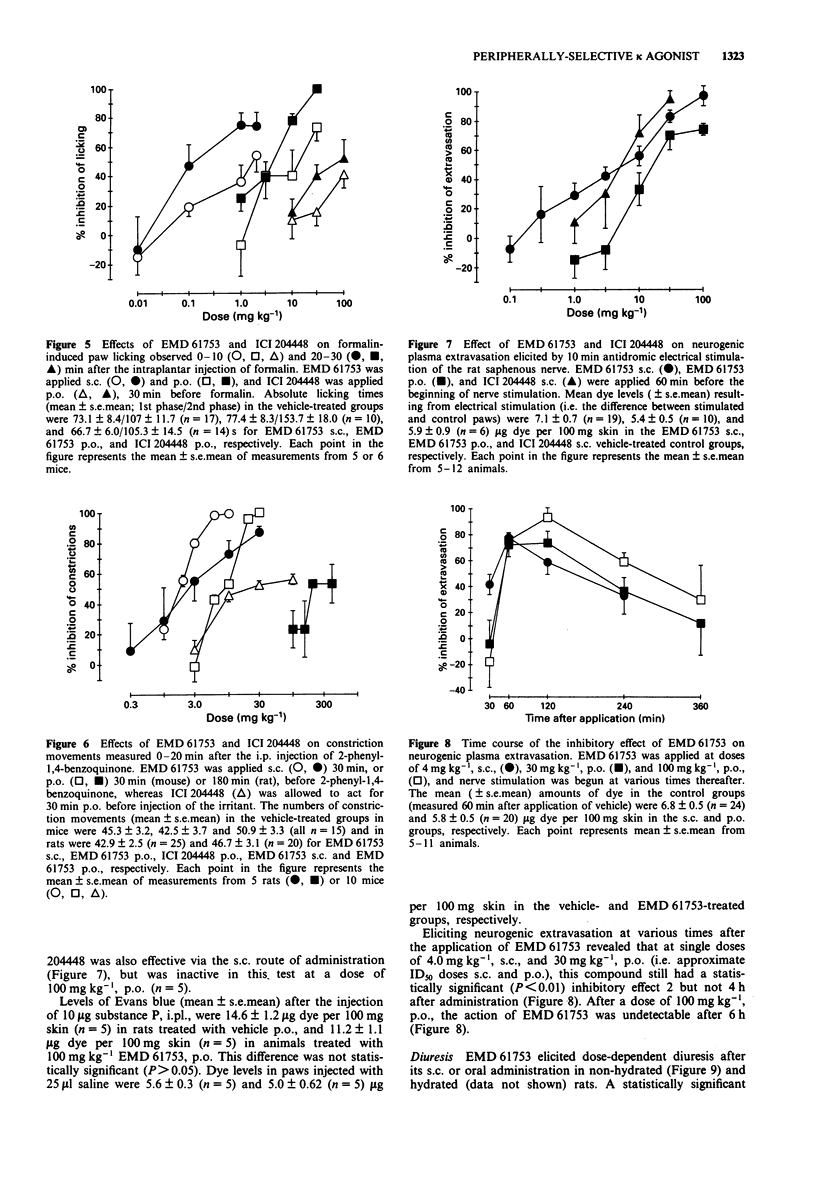
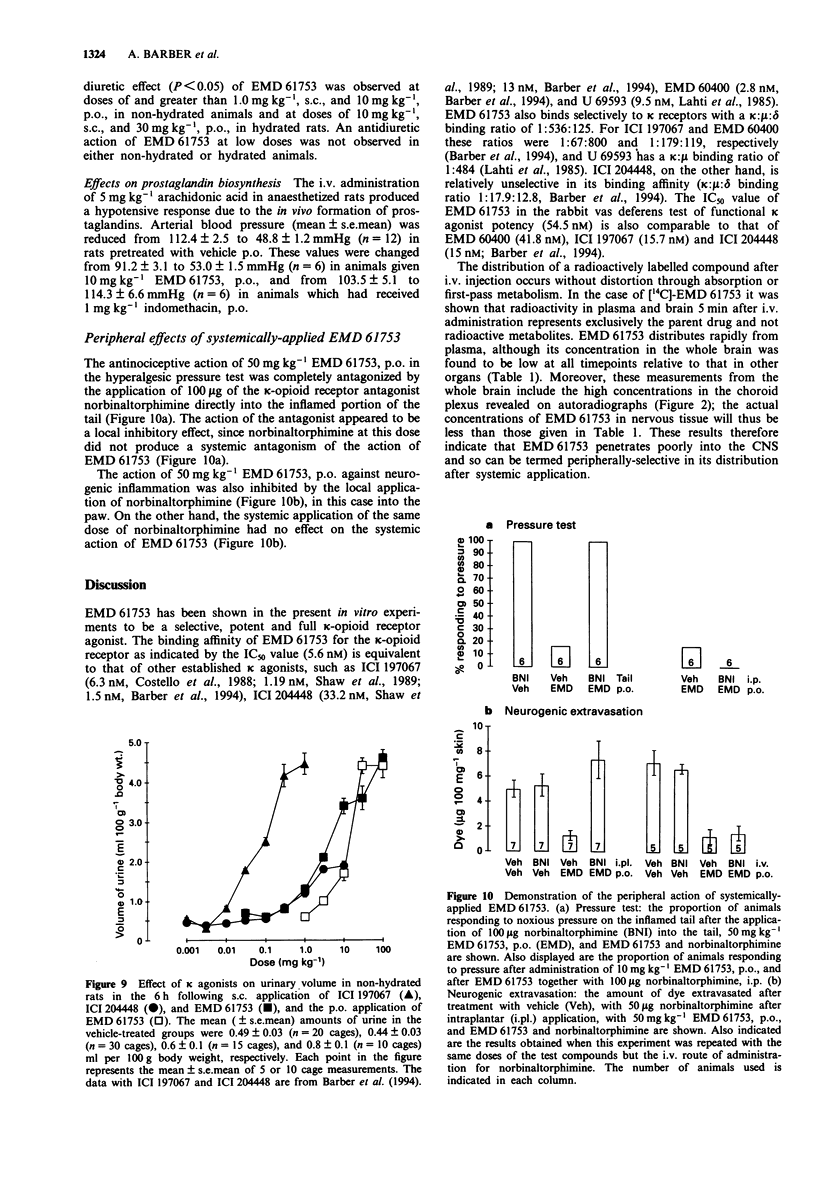
5 EMD 61753 produced dose-dependent, naloxone-reversible antinociception in the mouse formalin test (1st phase ID50 1.9 mg kg-1, s.c., and 10.4 mg kg-1, p.o.; 2nd phase ID50 0.26 mg kg-1, s.c., and 3.5 mg kg-1, p.o.) and rodent abdominal constriction test (ID50 mouse 1.75 mg kg-1, s.c., and 8.4 mg kg-1, p.o.; ID50 rat 3.2 mg kg-1, s.c., and 250 mg kg-1, p.o.). EMD 61753 was inactive, or only weakly effective, in the rat pressure test under normalgesic conditions. After the induction of hyperalgesia with carrageenin, however, this compound elicited potent, dose-dependent (ID50 0.08 mg kg-1, s.c., and 6.9 mg kg-1, p.o., after remedial application, and 0.2 mg kg-1, s.c., and 3.1 mg kg-1, p.o., after prophylactic application) and naloxone-reversible antinociception. The antinociceptive action of systemically-applied (50 mg kg-1, p.o.) EMD 61753 in the hyperalgesic pressure test was completely inhibited by injection of the κ-opioid antagonist norbinaltorphimine (100 μg) into the inflamed tissue, a result which indicates that this opioid effect is mediated peripherally.
6 Cutaneous plasma protein extravasation produced by antidromic electrical stimulation of the rat saphenous nerve was dose-dependently inhibited by systemically-applied EMD 61753 (ID50 values 3.7 mg kg-1, s.c., and 35.8 mg kg-1, p.o.), and this effect was completely antagonized by intraplantar application of norbinaltorphimine (50 μg). Extravasation elicited by the intraplantar application of substance P (10 μg) was not influenced by the administration of EMD 61753.
7 EMD 61753 produced dose-dependent diuresis in non-hydrated rats at doses of and above 1.0 mg kg-1, s.c., and 10 mg kg-1, p.o., and in saline-loaded rats at doses of and above 10 mg kg-1, s.c., and 30 mg kg-1, p.o.
8 The prostaglandin-mediated fall in mean arterial blood pressure elicited in anaesthetized rats by i.v. application of arachidonic acid was not inhibited by prior treatment with EMD 61753 (10 mg kg-1, p.o.). Thus, a blockade of prostaglandin synthesis via inhibition of cyclo-oxygenase activity does not contribute to the in vivo effects of EMD 61753 and its metabolites.
9 The present experiments therefore indicate that EMD 61753 is a potent, selective and orally-effective full κ-opioid receptor agonist which has a limited ability to penetrate the blood-brain barrier and elicit centrally-mediated sedation, putative aversion, diuresis, and antinociception. The inhibitory actions of systemically-applied EMD 61753 against hyperalgesic pressure nociception and neurogenic inflammation are mediated peripherally, probably by opioid receptors on the endings of sensory nerve fibres.
Keywords: EMD 61753, ICI 197067, ICI 204448, κ-opioid agonist, peripherally-selective, hyperalgesia, antinociception
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber A., Bartoszyk G. D., Greiner H. E., Mauler F., Murray R. D., Seyfried C. A., Simon M., Gottschlich R., Harting J., Lues I. Central and peripheral actions of the novel kappa-opioid receptor agonist, EMD 60400. Br J Pharmacol. 1994 Mar;111(3):843–851. doi: 10.1111/j.1476-5381.1994.tb14815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A., Gottschlich R. Opioid agonists and antagonists: an evaluation of their peripheral actions in inflammation. Med Res Rev. 1992 Sep;12(5):525–562. doi: 10.1002/med.2610120505. [DOI] [PubMed] [Google Scholar]
- Barber A. Mu- and kappa-opioid receptor agonists produce peripheral inhibition of neurogenic plasma extravasation in rat skin. Eur J Pharmacol. 1993 May 12;236(1):113–120. doi: 10.1016/0014-2999(93)90233-8. [DOI] [PubMed] [Google Scholar]
- Bentley G. A., Newton S. H., Starr J. Evidence for an action of morphine and the enkephalins on sensory nerve endings in the mouse peritoneum. Br J Pharmacol. 1981 Jun;73(2):325–332. doi: 10.1111/j.1476-5381.1981.tb10425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D. P., Giardina G., Gellai M., Dondio G., Edwards R. M., Petrone G., DePalma P. D., Sbacchi M., Jugus M., Misiano P. Opiate receptors within the blood-brain barrier mediate kappa agonist-induced water diuresis. J Pharmacol Exp Ther. 1993 Jul;266(1):164–171. [PubMed] [Google Scholar]
- Costello G. F., Main B. G., Barlow J. J., Carroll J. A., Shaw J. S. A novel series of potent and selective agonists at the opioid kappa-receptor. Eur J Pharmacol. 1988 Jul 14;151(3):475–478. doi: 10.1016/0014-2999(88)90546-8. [DOI] [PubMed] [Google Scholar]
- Di Chiara G., Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra L. A., Gmerek D. E., Winger G., Woods J. H. Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther. 1987 Aug;242(2):413–420. [PubMed] [Google Scholar]
- Follenfant R. L., Hardy G. W., Lowe L. A., Schneider C., Smith T. W. Antinociceptive effects of the novel opioid peptide BW443C compared with classical opiates; peripheral versus central actions. Br J Pharmacol. 1988 Jan;93(1):85–92. doi: 10.1111/j.1476-5381.1988.tb11408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley J., Ketchum S., Dickenson A. Peripheral kappa-opioid modulation of the formalin response: an electrophysiological study in the rat. Eur J Pharmacol. 1990 Dec 4;191(3):437–446. doi: 10.1016/0014-2999(90)94178-z. [DOI] [PubMed] [Google Scholar]
- Hunskaar S., Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987 Jul;30(1):103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Lahti R. A., Mickelson M. M., McCall J. M., Von Voigtlander P. F. [3H]U-69593 a highly selective ligand for the opioid kappa receptor. Eur J Pharmacol. 1985 Feb 26;109(2):281–284. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Manzanares J., Lookingland K. J., Moore K. E. Kappa opioid receptor-mediated regulation of dopaminergic neurons in the rat brain. J Pharmacol Exp Ther. 1991 Feb;256(2):500–505. [PubMed] [Google Scholar]
- Peters G. R., Ward N. J., Antal E. G., Lai P. Y., deMaar E. W. Diuretic actions in man of a selective kappa opioid agonist: U-62,066E. J Pharmacol Exp Ther. 1987 Jan;240(1):128–131. [PubMed] [Google Scholar]
- Pfeiffer A., Brantl V., Herz A., Emrich H. M. Psychotomimesis mediated by kappa opiate receptors. Science. 1986 Aug 15;233(4765):774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Risau W., Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990 May;13(5):174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- Rogers H., Birch P. J., Harrison S. M., Palmer E., Manchee G. R., Judd D. B., Naylor A., Scopes D. I., Hayes A. G. GR94839, a kappa-opioid agonist with limited access to the central nervous system, has antinociceptive activity. Br J Pharmacol. 1992 Aug;106(4):783–789. doi: 10.1111/j.1476-5381.1992.tb14413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell N. J., Schaible H. G., Schmidt R. F. Opiates inhibit the discharges of fine afferent units from inflamed knee joint of the cat. Neurosci Lett. 1987 Apr 23;76(1):107–112. doi: 10.1016/0304-3940(87)90201-1. [DOI] [PubMed] [Google Scholar]
- Seelig A., Gottschlich R., Devant R. M. A method to determine the ability of drugs to diffuse through the blood-brain barrier. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):68–72. doi: 10.1073/pnas.91.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried C. A., Adam G., Greve T. An automated direct-injection HPLC-method for the electrochemical/fluorimetric quantitation of monoamines and related compounds optimized for the screening of large numbers of animals. Biomed Chromatogr. 1986 Apr;1(2):78–88. doi: 10.1002/bmc.1130010206. [DOI] [PubMed] [Google Scholar]
- Shaw J. S., Carroll J. A., Alcock P., Main B. G. ICI 204448: a kappa-opioid agonist with limited access to the CNS. Br J Pharmacol. 1989 Apr;96(4):986–992. doi: 10.1111/j.1476-5381.1989.tb11911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg T. S., Bals-Kubik R., Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther. 1993 Apr;265(1):53–59. [PubMed] [Google Scholar]
- Smith T. W., Follenfant R. L., Ferreira S. H. Antinociceptive models displaying peripheral opioid activity. Int J Tissue React. 1985;7(1):61–67. [PubMed] [Google Scholar]
- Spanagel R., Herz A., Shippenberg T. S. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990 Nov;55(5):1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg. 1993 Jan;76(1):182–191. doi: 10.1213/00000539-199301000-00031. [DOI] [PubMed] [Google Scholar]
- Yonehara N., Imai Y., Chen J. Q., Takiuchi S., Inoki R. Influence of opioids on substance P release evoked by antidromic stimulation of primary afferent fibers in the hind instep of rats. Regul Pept. 1992 Mar 5;38(1):13–22. doi: 10.1016/0167-0115(92)90068-6. [DOI] [PubMed] [Google Scholar]