Abstract
The mechanism responsible for the protection against lethal organophosphate poisoning by pyridine-2-aldoxime methiodide (P-2-AM) was studied in the mouse. Two types of organophosphates were used: ethyl pyrophosphate (TEPP), E 600, Ro 3-0340, and Ro 3-0422 which form with true cholinesterase a diethylphosphoryl enzyme (1) and DFP, D 600, and Ro 3-0351 which form with true cholinesterase a diisopropylphosphoryl enzyme (2).
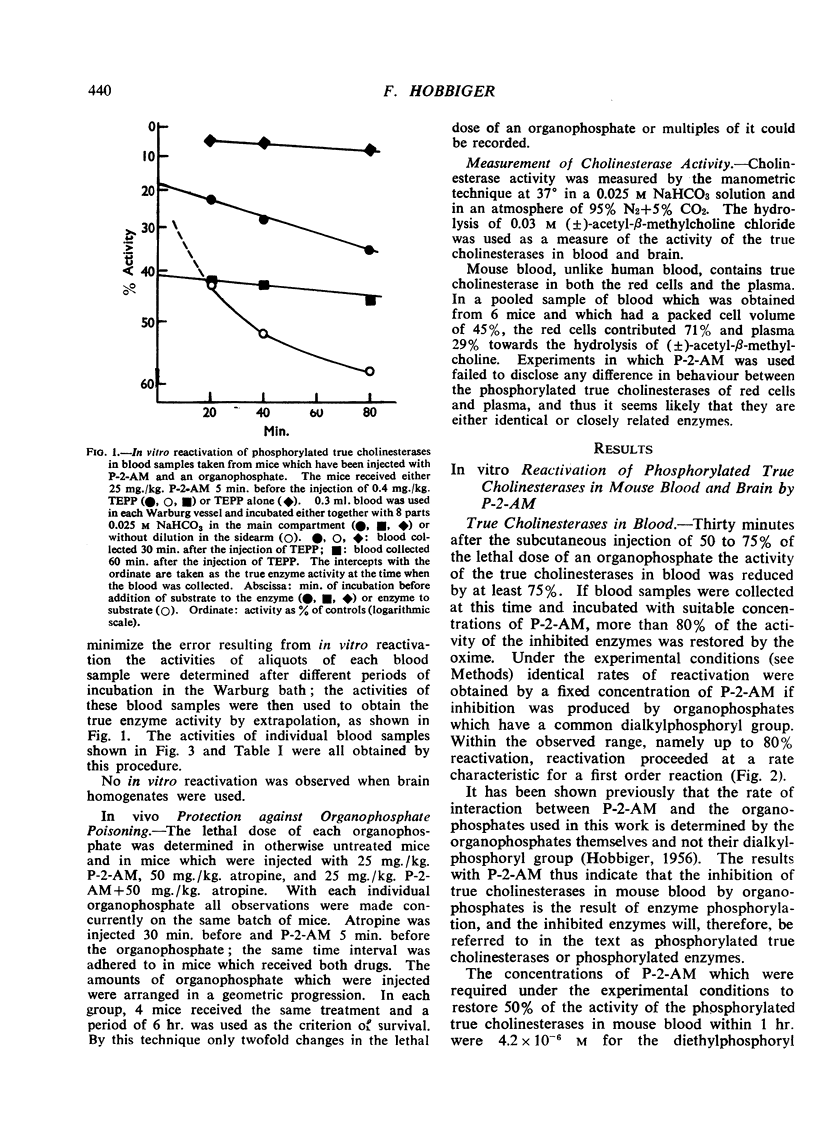
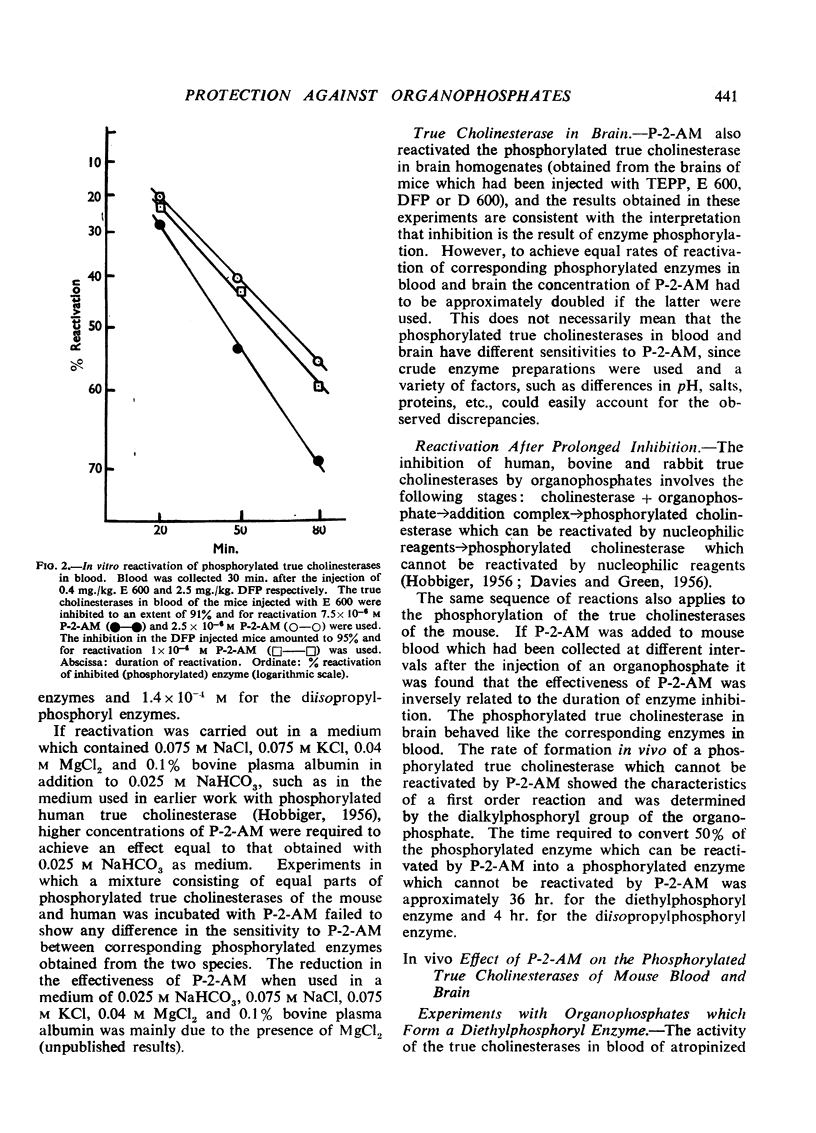
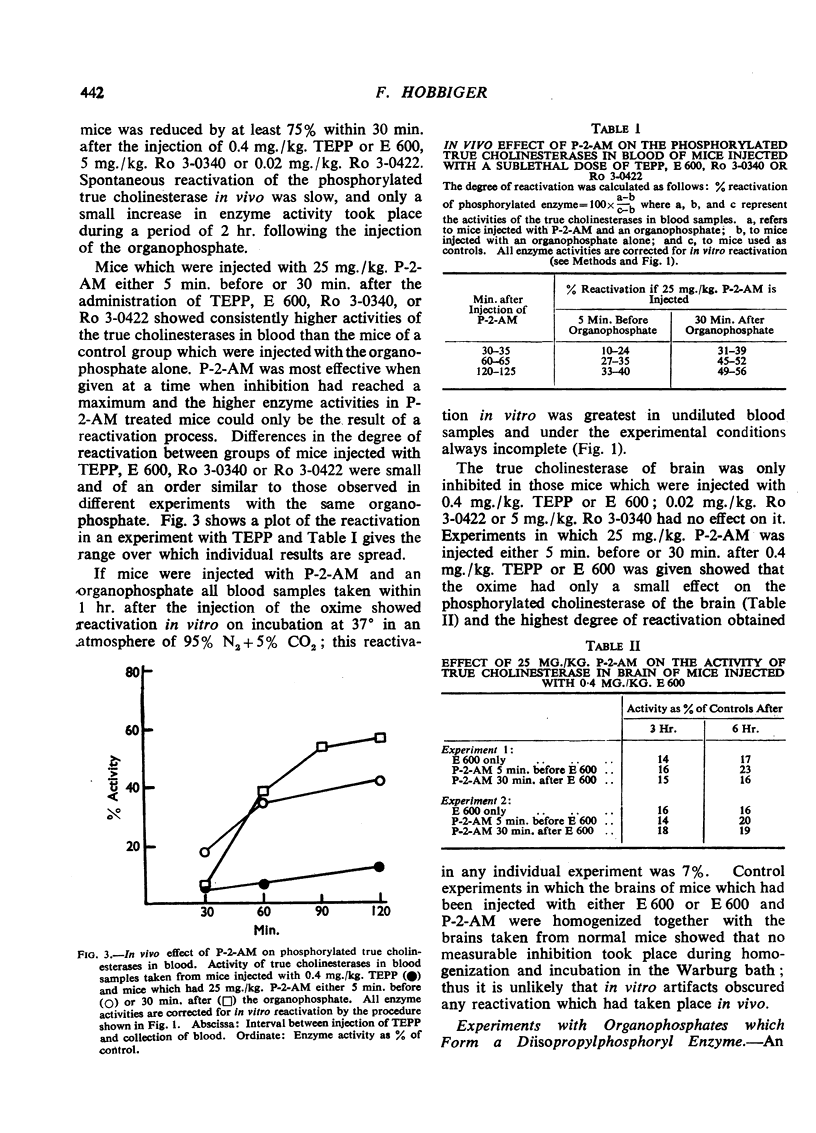
In vitro and under the experimental conditions used more than 50% reactivation of (1) was obtained within 1 hr. by concentrations of P-2-AM ranging from 0.5 to 1×10-5 M; 30 times higher concentrations of the oxime were required to achieve the same effect with (2). In vivo reactivation of phosphorylated true cholinesterases in blood amounted to 10 to 24% within the first 30 min. if 25 mg./kg. P-2-AM was injected (i.p.) 5 min. before a sublethal dose of TEPP, E 600, Ro 3-0340, or Ro 3-0422 and reactivation reached a maximum within 1 to 2 hr. after the injection of the oxime. P-2-AM was more effective when given 30 min. after the organophosphate. The effect of 25 mg./kg. P-2-AM on the phosphorylated true cholinesterase in brain (experiments with TEPP and E 600) was negligible. A dose of 25 mg./kg. P-2-AM had no consistent effect on the phosphorylated true cholinesterases in blood and brain of mice injected with sublethal doses of DFP, D 600, or Ro 3-0351.
The protection by 25 mg./kg. P-2-AM against lethal doses of TEPP, E 600, Ro 3-0422, and Ro 3-0340 was greater than that obtained with 50 mg./kg. atropine sulphate, but the degree of protection was determined by the organophosphate itself and not its dialkylphosphoryl group. Protection by 25 mg./kg. P-2-AM against lethal doses of DFP, D 600, and Ro 3-0351 was negligible. The antidotal effect of P-2-AM was potentiated by atropine. Mice which were injected with atropine and P-2-AM were protected to a greater extent against DFP than against Ro 3-0422, and protection against DFP was only slightly less than protection against TEPP. This is difficult to reconcile with a specific action of P-2-AM on phosphorylated cholinesterases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N., DAVISON A. N. The mechanism of inhibition of cholinesterases by organophosphorus compounds. Biochem J. 1953 Dec;55(5):763–766. doi: 10.1042/bj0550763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASKEW B. M., DAVIES D. R., GREEN A. L., HOLMES R. The nature of the toxicity of 2-oxo-oximes. Br J Pharmacol Chemother. 1956 Dec;11(4):424–427. doi: 10.1111/j.1476-5381.1956.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASKEW B. M. Oximes and hydroxamic acids as antidotes in anticholinesterase poisoning. Br J Pharmacol Chemother. 1956 Dec;11(4):417–423. doi: 10.1111/j.1476-5381.1956.tb00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A. S. V., HOBBIGER F. The inhibition of cholinesterases by alkyl-phosphates and alkylphenolphosphates. Br J Pharmacol Chemother. 1951 Dec;6(4):593–605. doi: 10.1111/j.1476-5381.1951.tb00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHILDS A. F., DAVIES D. R., GREEN A. L., RUTLAND J. P. The reactivation by oximes and hydroxamic acids of cholinesterase inhibited by organo-phosphorus compounds. Br J Pharmacol Chemother. 1955 Dec;10(4):462–465. doi: 10.1111/j.1476-5381.1955.tb00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN J. A., POSTHUMUS C. H. The mechanism of action of anti-cholinesterases. Acta Physiol Pharmacol Neerl. 1955;4(1):17–36. [PubMed] [Google Scholar]
- DAVIES D. R., GREEN A. L. The kinetics of reactivation, by oximes, of cholinesterase inhibited by organophosphorus compounds. Biochem J. 1956 Aug;63(4):529–535. doi: 10.1042/bj0630529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CANDOLE C. A., DOUGLAS W. W., EVANS C. L., HOLMES R., SPENCER K. E., TORRANCE R. W., WILSON K. M. The failure of respiration in death by anticholinesterase poisoning. Br J Pharmacol Chemother. 1953 Dec;8(4):466–475. doi: 10.1111/j.1476-5381.1953.tb01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CANDOLE C. A., McPHAIL M. K. Pentamethonium as an adjuvant to atropine in the therapy of paraoxon poisoning. Nature. 1954 Sep 18;174(4429):552–553. doi: 10.1038/174552a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., PATON W. D. M. The mode of action of tetraethyl pyrophosphate at the cat's neuromuscular junction. J Physiol. 1951 Dec 28;115(4):71–P. [PubMed] [Google Scholar]
- DUBOIS K. P., DOULL J., OKINAKA A. J., COON J. M. Studies on the toxicity and pharmacological actions of symmetrical and unsymmetrical diethyl bis (dimethylamido) pyrophosphate. J Pharmacol Exp Ther. 1953 Apr;107(4):464–477. [PubMed] [Google Scholar]
- GROBLEWSKI G. E., MCNAMARA B. P., WILLS J. H. Stimulation of denervated muscle by DFP and related compounds. J Pharmacol Exp Ther. 1956 Sep;118(1):116–122. [PubMed] [Google Scholar]
- HOBBIGER F. The inhibition of cholinesterases by 3-(diethoxyphosphinyloxy)-N-methylquinolinium methylsulphate and its tertiary base. Br J Pharmacol Chemother. 1954 Jun;9(2):159–165. doi: 10.1111/j.1476-5381.1954.tb00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES R., ROBINS E. L. The reversal by oximes of neuromuscular block produced by anticholinesterases. Br J Pharmacol Chemother. 1955 Dec;10(4):490–495. doi: 10.1111/j.1476-5381.1955.tb00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEWITZ H. A specific antidote against lethal alkyl phosphate intoxication. III. Repair of chemical lesion. Arch Biochem Biophys. 1957 Feb;66(2):263–270. doi: 10.1016/s0003-9861(57)80001-0. [DOI] [PubMed] [Google Scholar]
- KEWITZ H., NACHMANSOHN D. A specific antidote against lethal alkyl phosphate intoxication. IV. Effects in brain. Arch Biochem Biophys. 1957 Feb;66(2):271–283. [PubMed] [Google Scholar]
- KEWITZ H., NACHMANSOHN D., WILSON I. B. A specific antidote against lethal alkyl phosphate intoxication. II. Antidotal properties. Arch Biochem Biophys. 1956 Oct;64(2):456–465. doi: 10.1016/0003-9861(56)90288-0. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B., STEINER E. C. The cerebral distributions of a tertiary and a quaternary anticholinesterase agent following intravenous and intraventricular injection. J Pharmacol Exp Ther. 1956 Dec;118(4):420–434. [PubMed] [Google Scholar]
- LEWIS J. R., MCKEON W. B., Jr, LANDS A. M. Effect of various drugs in antagonizing the toxicity of TEPP. Arch Int Pharmacodyn Ther. 1955 Aug 1;102(4):371–390. [PubMed] [Google Scholar]
- MURTHA E. F., MCNAMARA B. P., EDBERG L. J., BERGNER A. D., WILLS J. H. Studies on the pharmacology of tetraethylpyrophosphate. J Pharmacol Exp Ther. 1955 Nov;115(3):291–299. [PubMed] [Google Scholar]
- PARKES M. W., SACRA P. Protection against the toxicity of cholinesterase inhibitors by acetylcholine antagonists. Br J Pharmacol Chemother. 1954 Sep;9(3):299–305. doi: 10.1111/j.1476-5381.1954.tb01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART W. C. Accumulation of acetylcholine in brain and blood of animals poisoned with cholinesterase inhibitors. Br J Pharmacol Chemother. 1952 Jun;7(2):270–276. doi: 10.1111/j.1476-5381.1952.tb01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON I. B., GINSBURG B. A powerful reactivator of alkylphosphate-inhibited acetylcholinesterase. Biochim Biophys Acta. 1955 Sep;18(1):168–170. doi: 10.1016/0006-3002(55)90040-8. [DOI] [PubMed] [Google Scholar]


